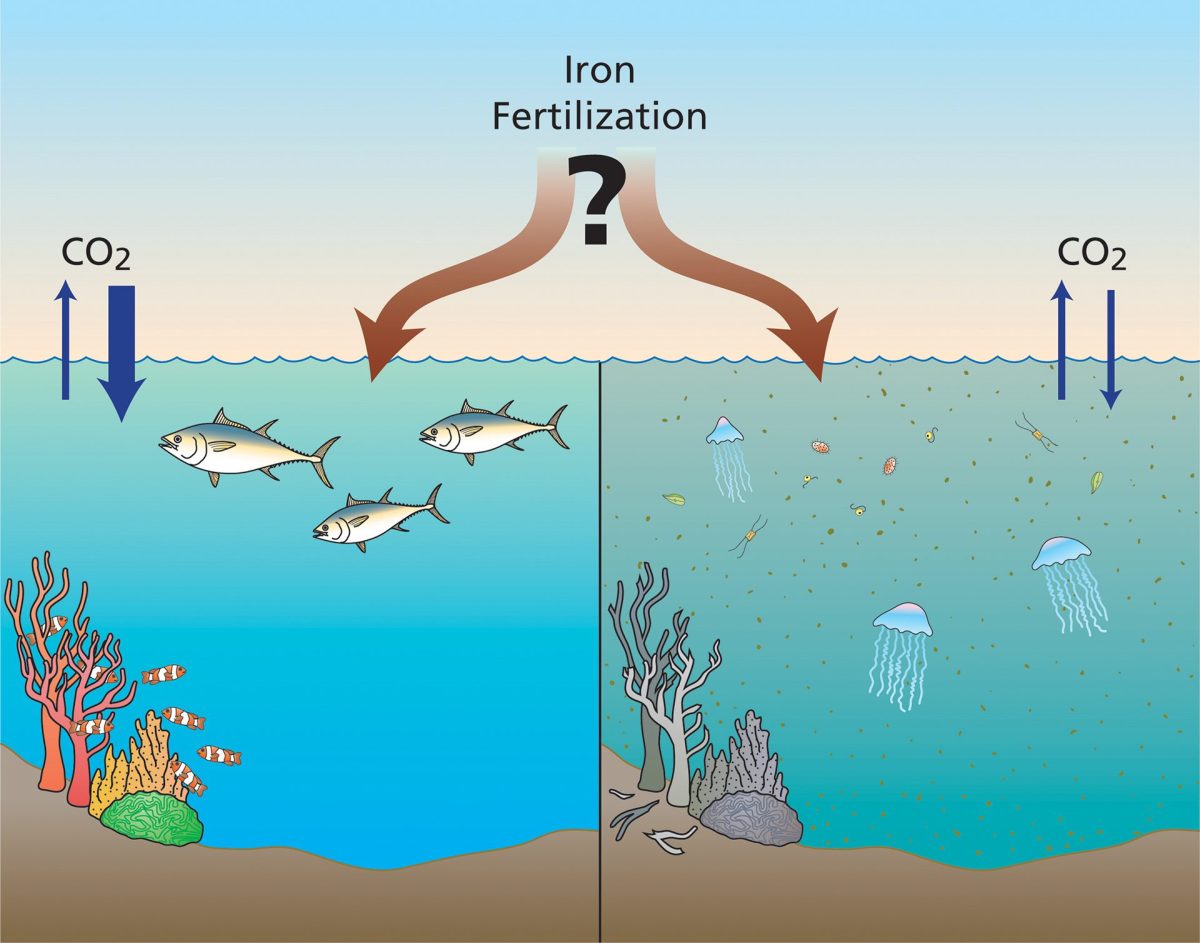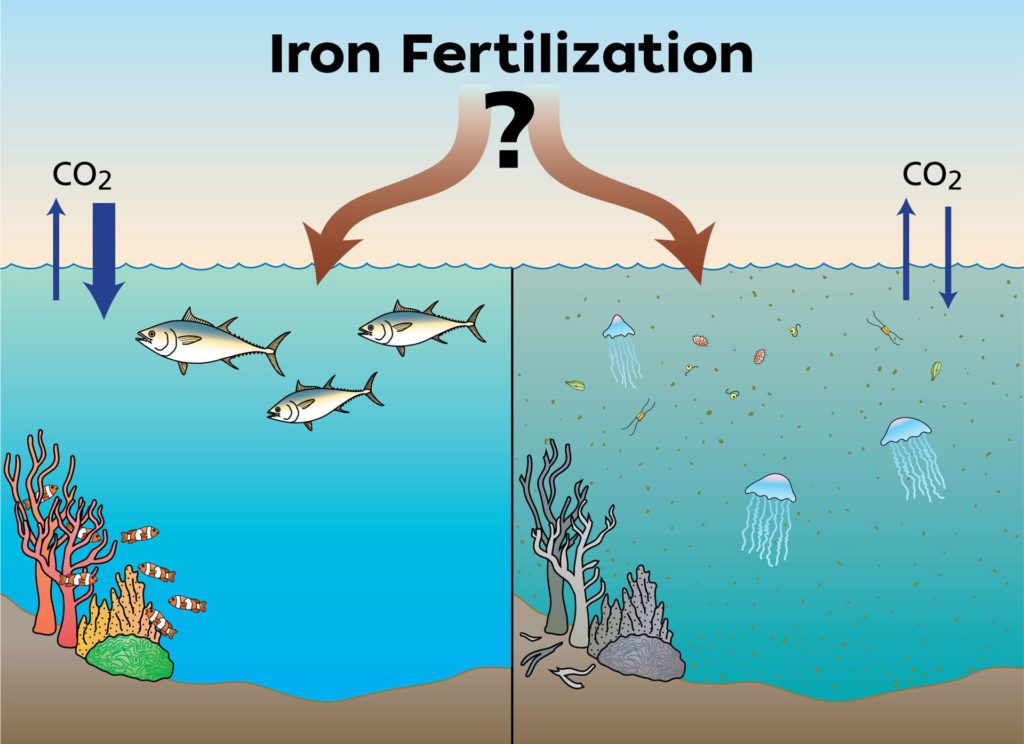
Iron fertilization is a Carbon Dioxide Removal (CDR) technique that would artificially add iron to the ocean’s surface to stimulate growth of phytoplankton. Scientists are researching the viability of this technique to mitigate climate change. Illustration by Jack Cook, ©Woods Hole Oceanographic Institution
What is iron fertilization?
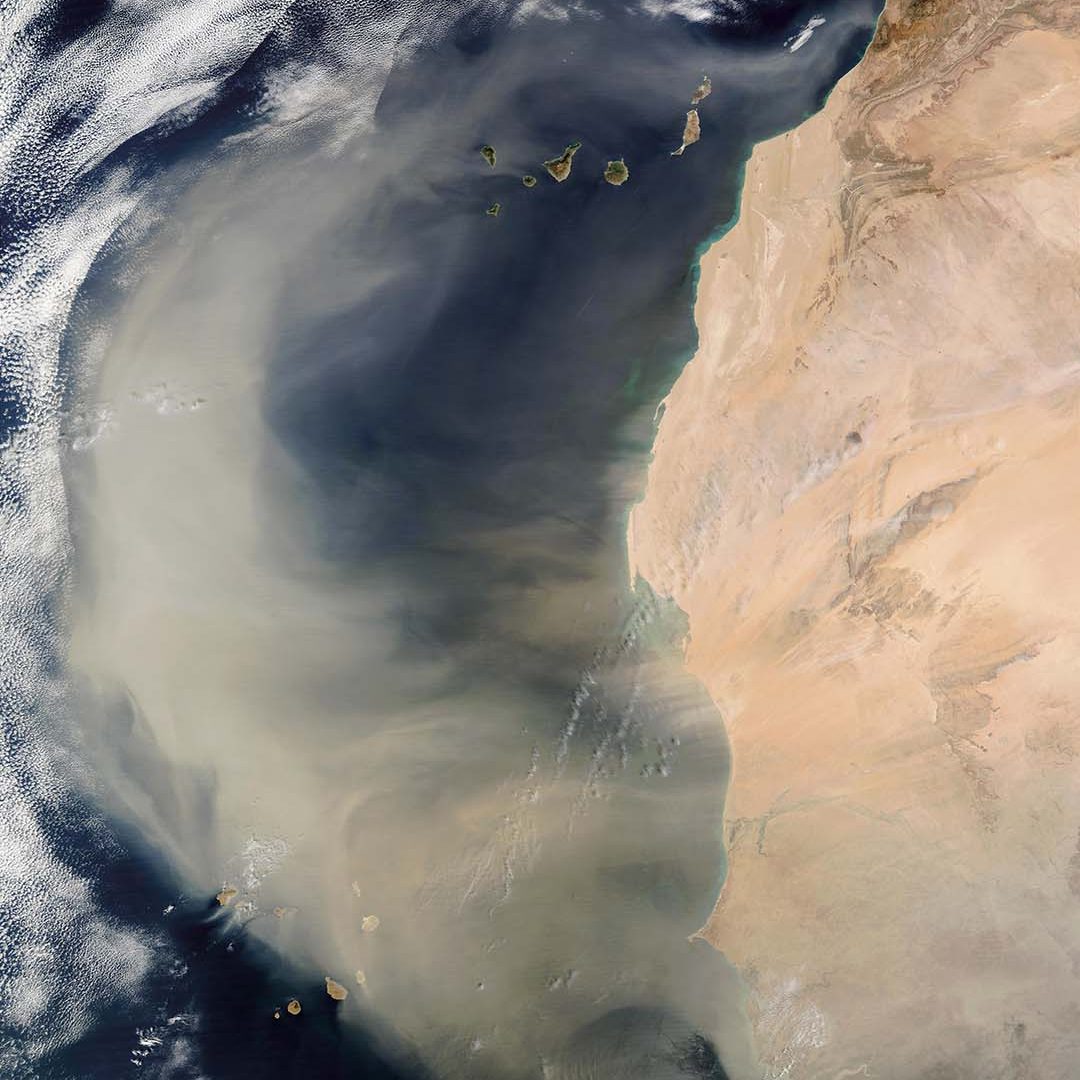
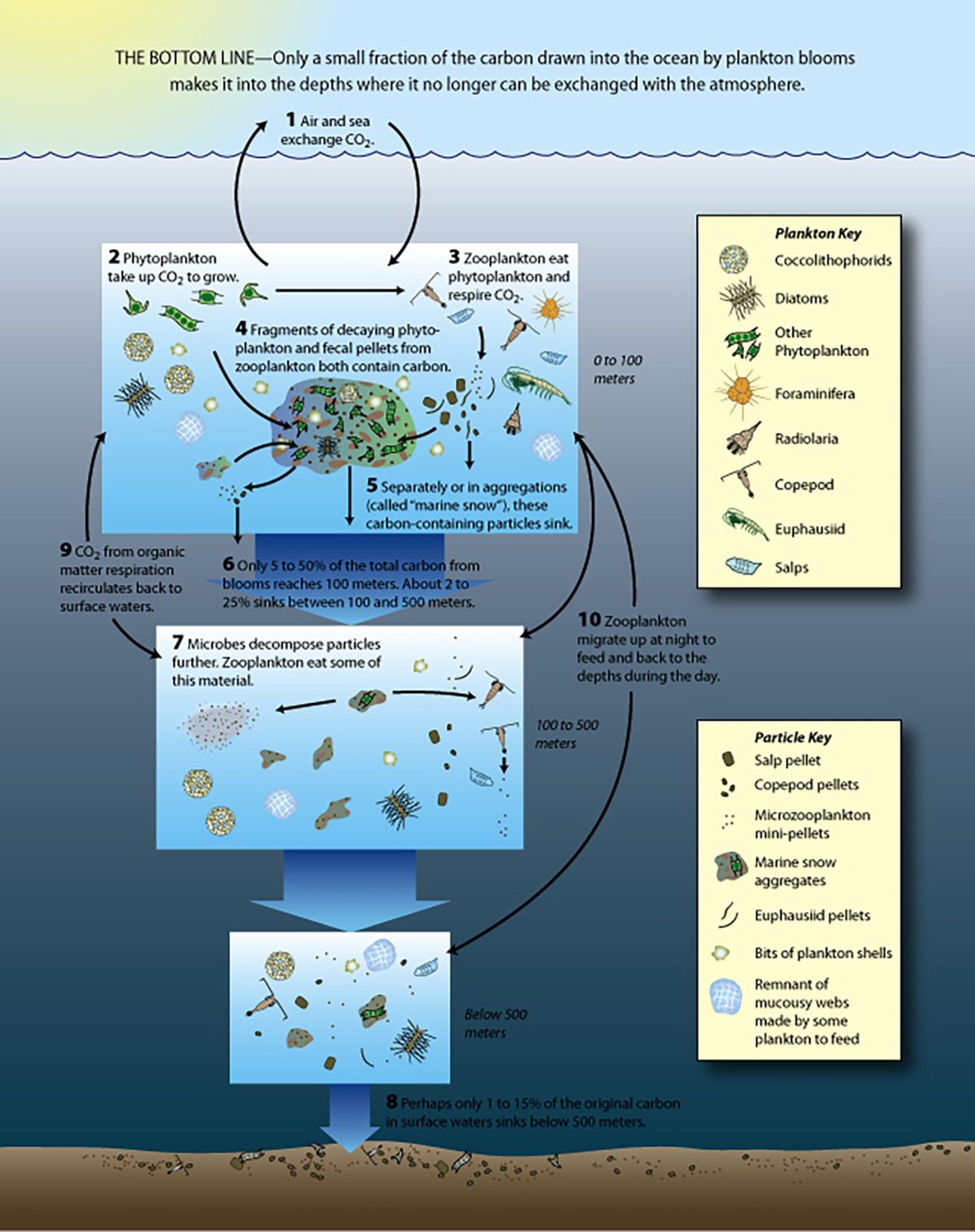
All organisms require nutrients to live and grow, and those living in the open ocean are no exception. Tiny phytoplankton drift on the water’s surface, converting sunlight, water, and carbon dioxide from the atmosphere into food and oxygen. Like other photosynthetic organisms, such as land plants, these tiny algae and cyanobacteria require nutrients for photosynthesis to occur. On land, nitrogen and phosphorous are often the limiting nutrients, but in many parts of the open ocean, these nutrients are generally available. It’s other, trace nutrients that are missing, and iron is one of the key players in this system. Adding small amounts of iron to the ocean’s surface can trigger a bloom of phytoplankton big enough to be seen from space. Such additions happen naturally, such as when winds blow dust from the Sahara Desert or ash from a volcanic eruption. When the plume of dust or ash settles over the ocean’s surface, it triggers massive blooms of phytoplankton that remove substantial amounts of carbon dioxide from the atmosphere. Iron fertilization is a Carbon Dioxide Removal (CDR) technique that would mimic this natural system, artificially adding iron to the ocean’s surface to stimulate growth of phytoplankton.
Why could iron fertilization be important for climate change?
Because iron is a micronutrient, phytoplankton need only trace amounts for it to have a massive impact. Ash from the 2008 eruption of Kasatochi in the Aleutian Islands created an algal bloom that is estimated to have removed 10 million tons of carbon from the sunlit zone. Soot from the 2019-2020 Australian wildfires caused a bloom between New Zealand and South America that may have removed as much as 150 to 300 million tons of carbon.
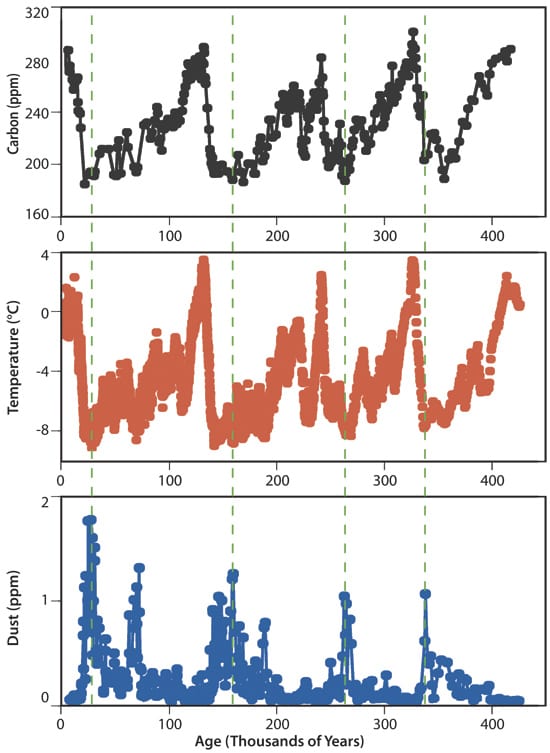
Ice core records also show a link between iron-rich dust and changes in climate. During times when large amounts of dust settled over the ocean, global temperatures dropped, with an estimated 60 billion tons of carbon drawn out of the atmosphere during these events. Many scientists argue that the iron dust contributed, at least in part, to the development of past glacial periods.
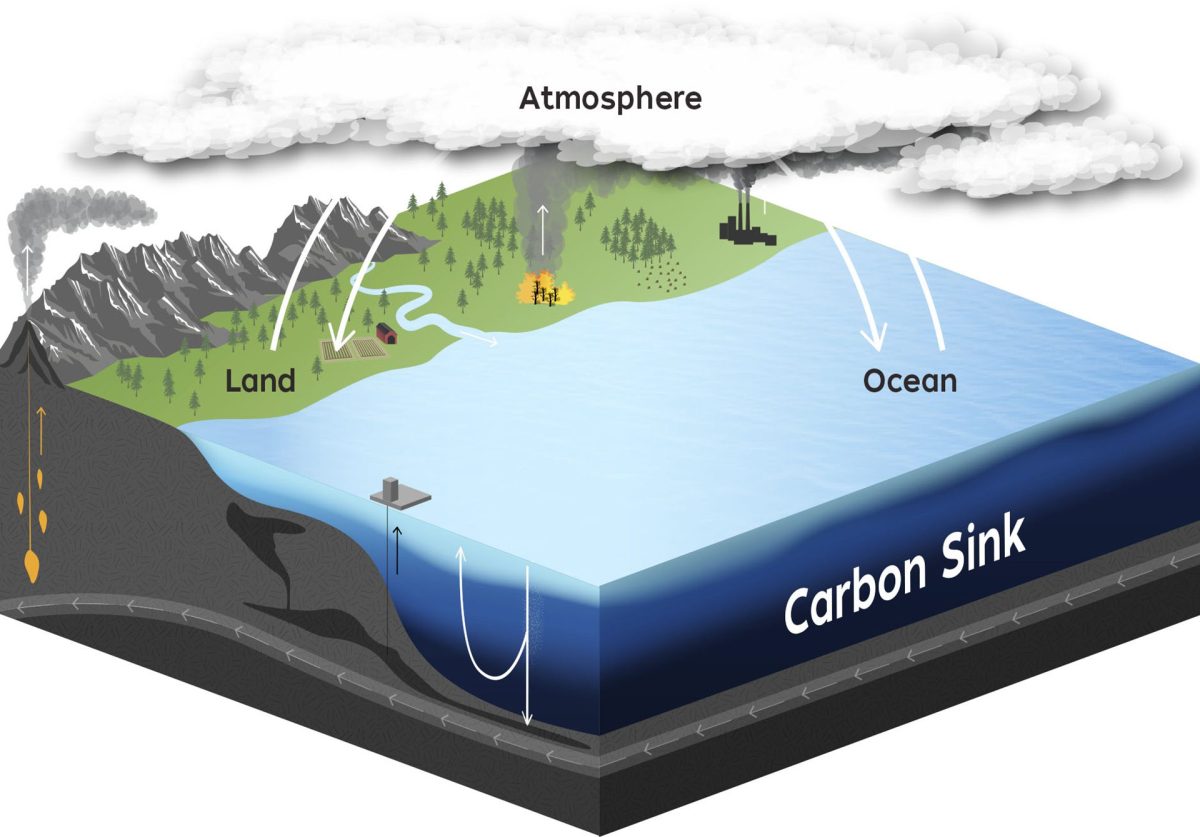
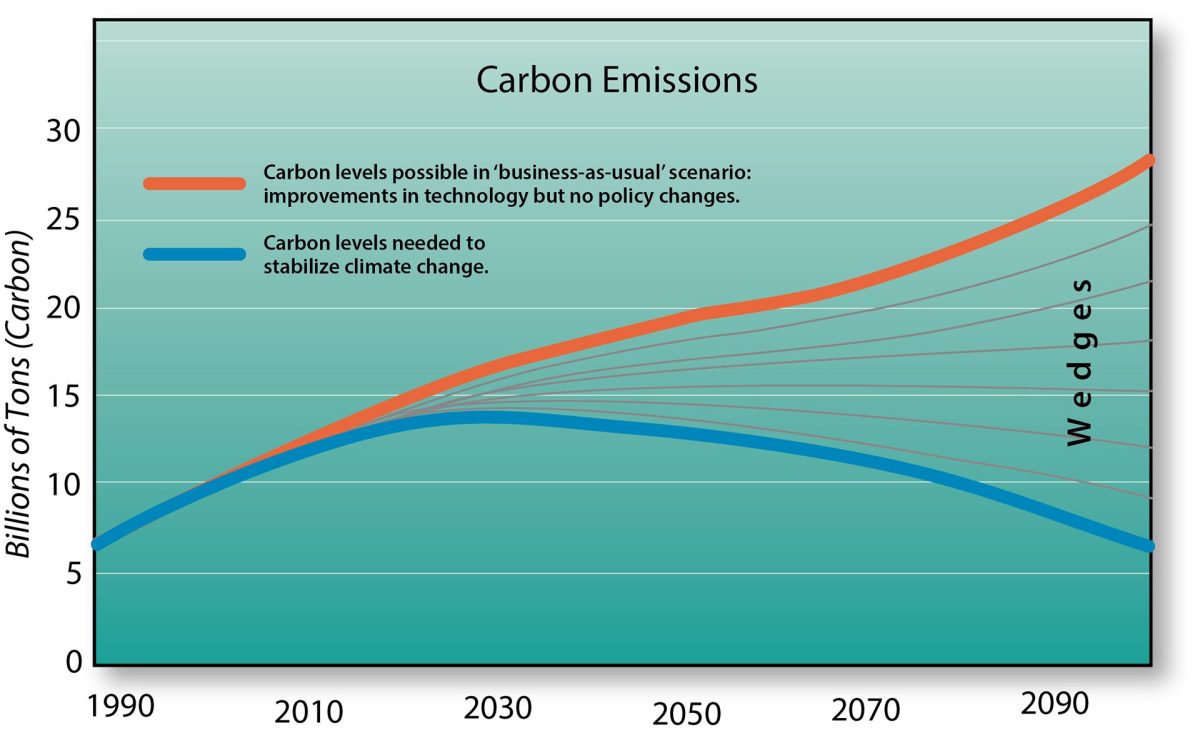
If relatively small amounts of iron can be added to the ocean’s surface to effectively remove large amounts of carbon dioxide from the atmosphere, iron fertilization has the potential to play a pivotal role in reducing additional impacts associated with climate change. But it will only work if the carbon removed from the atmosphere sinks to the ocean depths, where it will be locked away for at least a century. This would buy time for a full transition from fossil-fuels to renewable sources of energy.
What are ocean scientists doing to employ iron fertilization?
In the 1990s and early 2000s, a series of experiments tested iron fertilization in the open ocean. These tests consistently found that adding iron led to phytoplankton blooms, However, the extent to which that carbon sank to the depths wasn’t always measured, and the phytoplankton were not able to use all of the iron for growth before the mineral sank.
Researchers did document changes in phytoplankton communities, with diatoms becoming more abundant than many other types of phytoplankton. These algae can be up to 1,000 times larger than cyanobacteria, allowing them to take up more carbon dioxide via photosynthesis. Diatoms create silica-based glass-like shells that add weight, increasing the likelihood that they will sink faster than other, smaller phytoplankton when they die. Their fast growth rates and loss to the deep sea bodes well for potential removal of carbon from the atmosphere and its sequestration deeper in the ocean.
Some diatoms, however, release toxins that can contribute to harmful algal blooms, although these did not occur following any of the field experiments. In addition, iron fertilization has the potential to alter where and how nutrients are allocated in the marine ecosystem. Until experiments are done to test these potential outcomes and determine how much carbon can be sequestered in the ocean depths, iron fertilization should not be put to use as a method of slowing climate change.
Early iron fertilization experiments faced resistance, due to the many unknowns. Despite this, scientists are returning to the idea as one CDR tool that should be on the table in the fight against climate change. They are currently working to create codes of conduct, so that research can be conducted in a transparent manner to better understand both intended and unintended consequences of adding iron to the ocean’s surface.
New technologies using autonomous platforms and sensors now exist that allow scientists to fully investigate the potential for iron to remove atmospheric carbon and track the subsequent movement of that carbon through the ocean. Because iron fertilization would be relatively inexpensive, it could be an important part of a suite of CDR activities aimed at removing excessive amounts of carbon dioxide from our atmosphere. But it’s important to remember that such approaches do not replace the need for immediate and major reductions in the use of fossil fuels that produce carbon dioxide in the first place.
Bach, L.T. et al. CO2 removal with enhanced weathering and ocean alkalinity enhancement: Potential risks and co-benefits for marine pelagic ecosystems. Frontiers in Climate. October 11, 2019. doi: 10.3389/fclim.2019.0007.
Buesseler, Ken. Personal communication.
Burt, D.J. et al. The sensitivity of the marine carbonate system to regional ocean alkalinity enhancement. Frontiers in Climate. July 9, 2021. doi: 10.3389/fclim.2021.624075.
Castañón, Laura. An ocean of opportunity. Oceanus. December 7, 2021. https://www.whoi.edu/oceanus/feature/an-ocean-of-opportunity/
Doney, S.C. et al. A research strategy for ocean-based carbon dioxide removal and sequestration. Consensus Study Report Highlights. December 2021. https://www.nap.edu/resource/26278/Ocean_CDR_2021.pdf
Federer, A. et al. Assessing the influence of ocean alkalinity enhancement on a coastal phtyoplankton community. Biogeosciences. Preprint. 2022. doi: 10.5194/bg-2022-17.
Lubofsky, Evan. The ocean has a serious case of heartburn. Is relief on the way? Oceanus. July 1, 2021. https://www.whoi.edu/oceanus/feature/ocean-alkalinity/