
The ocean has heartburn. Is relief on the way?
Researchers investigate the use of alkalinity enhancement to quell ocean acidification and help maintain the sea’s role as a carbon sink
Estimated reading time: 5 minutes
The Earth's atmosphere contains more carbon dioxide (CO2) than at any time in the last 20 million years, researchers say. The levels would be even higher if it weren't for the ocean, which slurps up carbon emissions and stores roughly 60 times more carbon than the atmosphere. But high carbon levels in the ocean are causing the pH of seawater to drop and ocean acidity to rise. This phenomenon, known as ocean acidification, has given our top ally in the fight against climate change a serious case of heartburn.
Marine organisms are feeling the effects. Corals, clams, snails, and other organisms continue to build their shells and skeletons, but building them quickly enough, or thick enough, is getting more challenging as seawater becomes more corrosive. The changes in water chemistry are also reducing the amount of carbonate ions in the ocean-the primary building blocks of shell building.
Fortunately, the weathering of rocks on land produces alkalinity, which has an "antacid" effect once it washes into the ocean. Researchers estimate there is currently the equivalent of 200 billion antacid tablets in the global ocean. Alkalinity also plays a critical role in helping the ocean store carbon. But there's a problem: alkalization of the ocean from the natural geological cycle is slow, sometimes taking hundreds of thousands of years to occur.
Adam Subhas, a marine chemist at WHOI, wants to speed up the process. He is investigating the viability of an ocean intervention-based process known as ocean alkalinity enhancement, which he loosely describes as adding "Tums" to the ocean.
"Ocean alkalinity enhancement has the potential to offset the effects of ocean acidification by increasing pH levels and neutralizing the CO2 building up in the water," he says. "And, it could help to mitigate anthropogenic CO2 emissions by increasing the buffering capacity of the global ocean."
Before we can start alkalinizing the ocean, however, we need to understand the impacts it will have on marine life.
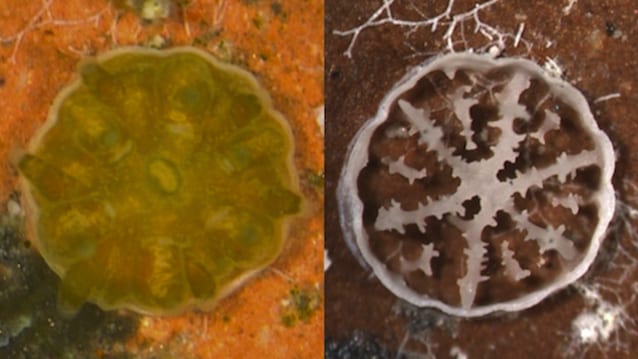
A three-week-old coral polyp (left), and its delicate skeleton (right), demonstrate how corals respond to increasing ocean acidification, which can impair their ability to build their calcium carbonate skeletons. (Photo by Elizabeth Drenkard, © Woods Hole Oceanographic Institution)
Subhas and colleagues from the National Oceanography Centre and the University of Portsmouth in the UK have been running experiments to investigate potential impacts. Last year, the team collected seawater samples from open-ocean sites near the Bahamas and in the central North Atlantic Ocean. The samples included shell-building organisms significantly smaller in size than clams and snails-such as plankton and amoebas. The researchers added precise mixtures of sodium bicarbonate and sodium carbonate-baking soda, essentially-to 10 liter incubation containers of seawater in varying amounts: low, medium, and high. They also added an isotope tracer, which allowed them to see how or if the organisms take up carbon and incorporate it into their structures.
"We need to measure the community's biological response so we can determine if, for example, the alkalinity is causing the organisms to grow more, if there's more carbon uptake, or if there's no response at all," Subhas says.
B.B. Cael, a biogeochemist at the National Oceanography Centre, says there's very little understanding in the scientific community about how natural marine biological communities respond to increased alkalinity levels. He and Subhas began collaborating on ocean alkalinity enhancement after participating in the NSF-EAGER Chief Scientist Training Cruise on the R/V Kilo Moana in June, 2019.
"We're constantly surprised by the way life responds to things," Cael says. "So, getting a sense for what the biological response will be, and if there's a threshold in terms of the amount of alkalinity we could put into the ocean without causing a response, is really important."

As part of the incubation experiments at sea, 10 liter containers filled with natural seawater and added alkalinity were used (in the foreground). These containers were incubated in the large blue tanks in the background, which held the them at a sea surface temperature and light level that would be found at water depth of roughly 15 meters. The circular disks in the foreground are filtering units that sampled the particulate material in the incubations once they were completed. (Photo by Ashley Maloney, Princeton University)
The researchers hope to avoid unintended consequences. For example, if alkalinity enhancement causes algae in surface waters to photosynthesize less-which is a possibility-it could cause a negative climate feedback loop by impairing the ocean's ability to soak up as much CO2 from the atmosphere. Another unintended consequence could occur, Subhas says, if organisms start using the alkalinity to build thicker-than-normal shells. This would leave the ocean less alkaline, which could interfere with its ability to absorb atmospheric carbon emissions.
The researchers have been running the seawater samples through a range of analytical tools to measure total alkalinity and carbon. Their analysis is in the early stages, but so far, exposure to high amounts of alkalinity appears to have different effects at the two open-ocean sites. At the site closer to the coast, there is little to no effect, even at very high alkalinity loads. But toward the middle of the North Atlantic where there's less biological productivity, high doses appear to inhibit the growth of plankton, which causes them to take up less carbon.
"The alkalinity could be having a destabilizing effect on the natural community," Subhas says, "but whether the effect is long-term, or if the ecosystem can bounce back quickly, are open questions." On a broad scale, this could have ramifications for the carbon cycle since smaller-sized plankton don't weigh as much, and when they die, they may not sink deep enough to bury carbon in the seafloor.
In the next phase of their research, Subhas and Cael hope to double the number of incubations and use larger, 100 liter containers in order to collect more types of organisms. And because they've learned that different ecosystems will likely respond differently to increased alkalinity, they want to run experiments in as many parts of the global ocean as is practical.
"Eventually, we hope to be able to disperse alkalinity off the back of a ship," Subhas says. "The payoff is potentially huge, but the only way we're going to get there is with dedicated investment and demonstrating this as a solution. Any technology that is proposing to operate at relevant scales needs to overcome this question of what's the ocean ecosystem going to do. If it's going to screw up the ocean, no one's going to do it."
Cael agrees. "We believe the theory and we want it to have the effect, but there's a lot of complex biology that sits between theory and practice. Going from the lab to reality will be really important steps here," he says.
This work is funded by Assistant Scientist Support Funding (ASES), and Subhas’s Startup package.




