In this section
Ocean Topics
- Climate & Weather
- How the Ocean Works
- Ocean & Human Lives
- Ocean Life
- Sustainable Ocean
- Ocean Tech
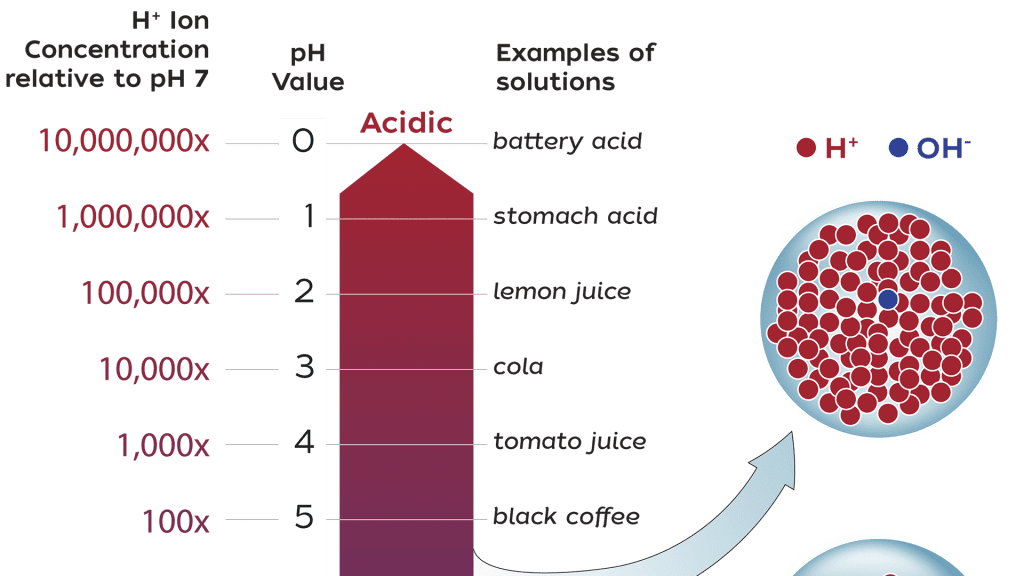
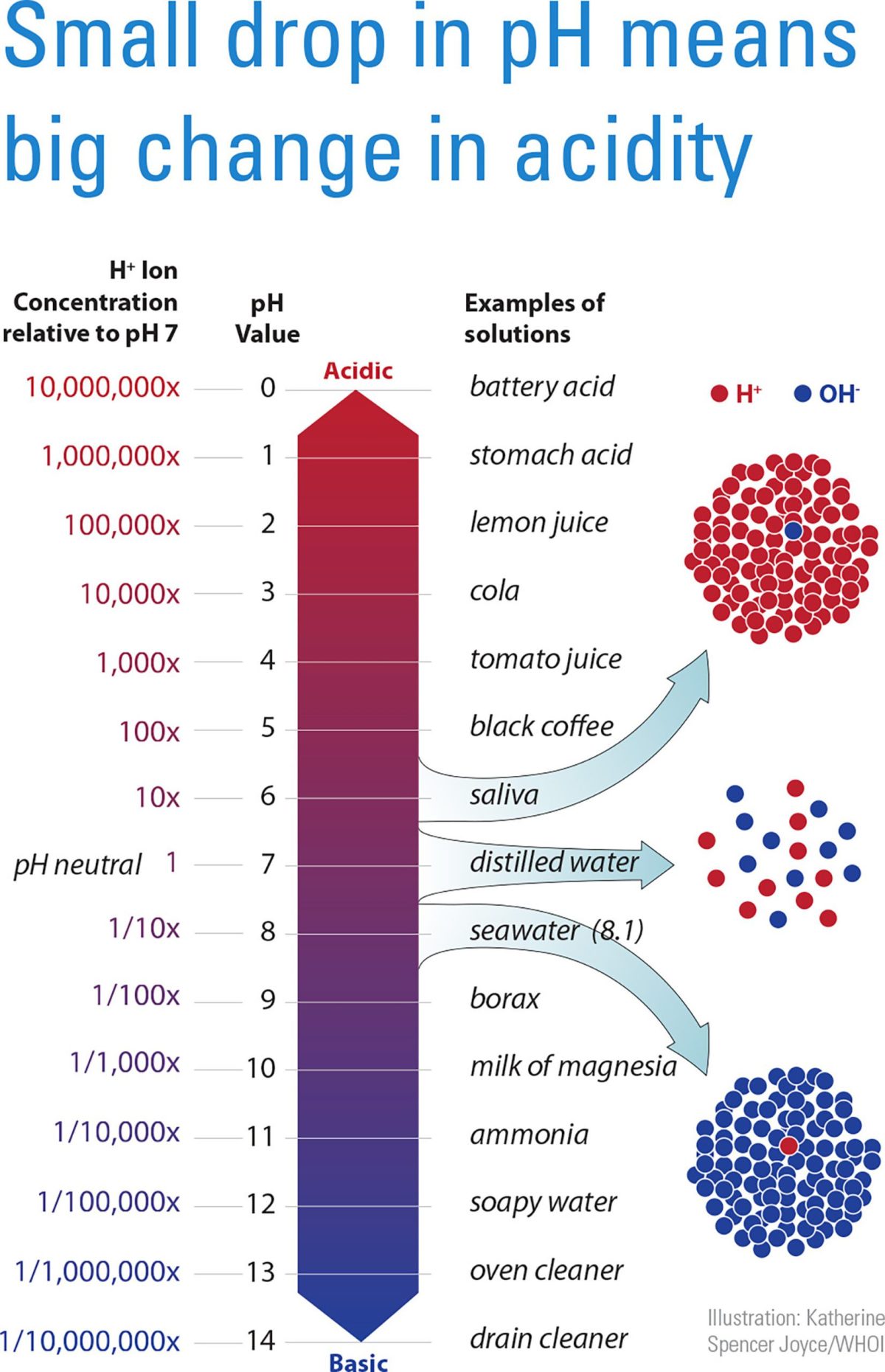
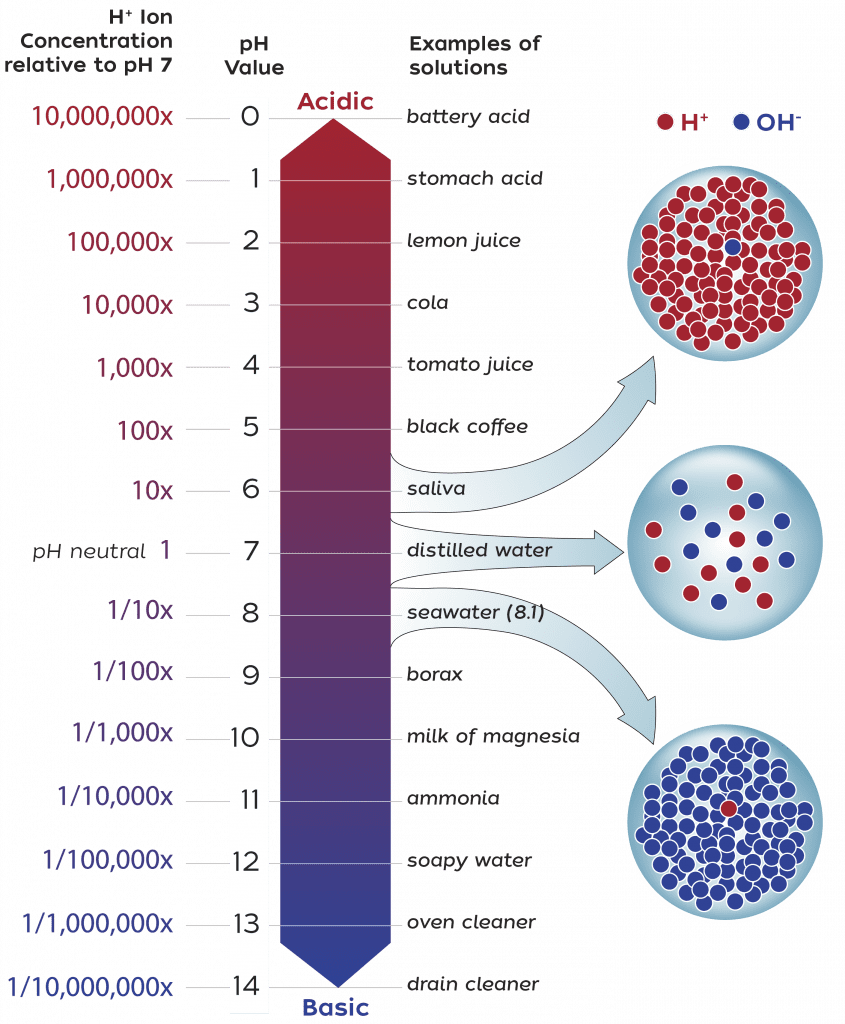
pH is a measure of the concentration of hydrogen ions in a solution. The more hydrogen ions that are present, the more acidic is the solution. The pH scale ranges from zero (very acidic) to 14 (very basic).
A pH of 7 is neutral, a pH less than 7 is acidic, and a pH greater than 7 is basic. For example, the pH of lemon juice is 2 while some antacids have a pH of about 10.
Ocean water is normally slightly basic, with a surface-water pH of about 8.2, but that has declined in recent years to about 8.1. A decrease of 0.1 pH units may not seem like much, but because the pH scale is logarithmic, each unit on the pH scale represents a tenfold change in acidity.
For example, water with a pH of 6 is ten times more acidic than neutral water with a pH of 7, and 100 times more acidic than water with a pH of 8. So a decrease of 0.1 pH units represents a 26 percent increase in the relative acidity of ocean water.
Articles Related to The pH Scale
From Oceanus Magazine
To Tag a Squid
How Do Corals Build Their Skeletons?
Searching for ‘Super Reefs’
Coral Crusader
Hidden Battles on the Reefs
Is Ocean Acidification Affecting Squid?
Swimming in Low-pH Seas
Can Squid Abide Ocean’s Lower pH?
News Releases
Ocean acidification causing coral ‘osteoporosis’ on iconic reefs
Scientists Pinpoint How Ocean Acidification Weakens Coral Skeletons
Climate Change Will Irreversibly Force Key Ocean Bacteria into Overdrive
Acidifying oceans could spell trouble for squid
WHOI to Host Public Event on Ocean Acidification
News & Insights
WHOI working to address ocean acidification; protect region’s vital shellfish industry
Working from Home: Mallory Ringham
Kalina Grabb studies some of the ocean’s most reactive chemicals
The oceans are losing oxygen, and faster than we thought
Falling in love with foraminifera
Putting the ‘nuclear coffin’ in perspective
WHOI in the News
The Top Eight Ocean Stories of 2022
The $500 Billion Question: What’s the Value of Studying the Ocean’s Biological Carbon Pump?
Ecology Research: Ocean acidification causing coral ‘osteoporosis’ on iconic reefs
Disentangling influences on coral health
Coral develops ‘osteoporosis’ because of acidic oceans caused by climate change, study reveals
Ocean acidification causing coral ‘osteoporosis’ on iconic reefs
Panel delves into impact of ocean acidification
Features

Dead zones occur when the water lacks oxygen. Like us, marine animals require oxygen to breathe, and when oxygen levels…

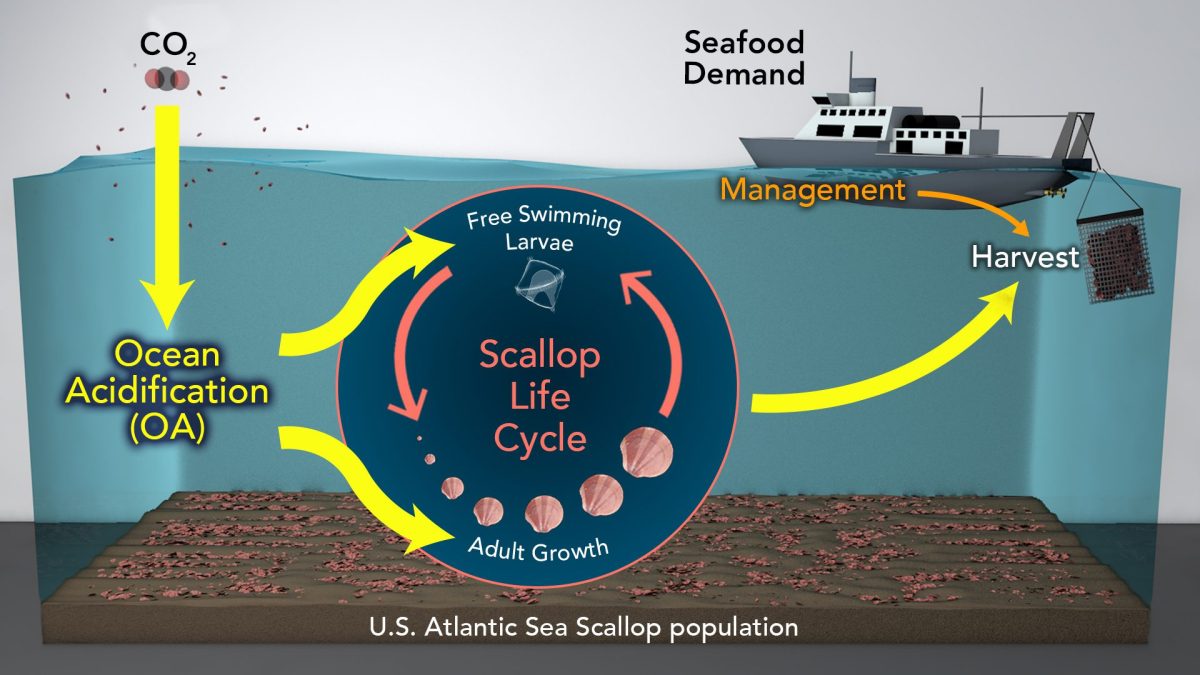
Ocean acidification is a reduction in the pH of the ocean over an extended period of time, caused primarily by…
Biogeochemistry studies the cycles of crucial elements, such as carbon and nitrogen, and their interactions with other substances and organisms…

When carbon dioxide mixes with seawater it has the effect of reducing the availability of carbonate ions, which many marine…

These questions were widely distributed to the research community with the request to draft concise replies summarizing current knowledge with…