
How Do Corals Build Their Skeletons?
Subtle architecture affects reefs' ability to withstand threats
Estimated reading time: 7 minutes
Corals are under a lot stress these days—from pollution, overfishing, sea level rise, warmer seawater temperatures, and the increasing acidity of the oceans. Among these stressors, the impact of ocean acidification is often the most insidious and difficult to detect. It threatens coral reefs by making it harder for corals to build their skeletons. But exactly how do corals go about growing their skeletons?
Nathaniel Mollica, a graduate student in MIT-WHOI Joint Program, was part of a team that delved into the details of how coral skeletons are built. What they found will help scientists predict more precisely how corals throughout the world will fare under ocean acidification.
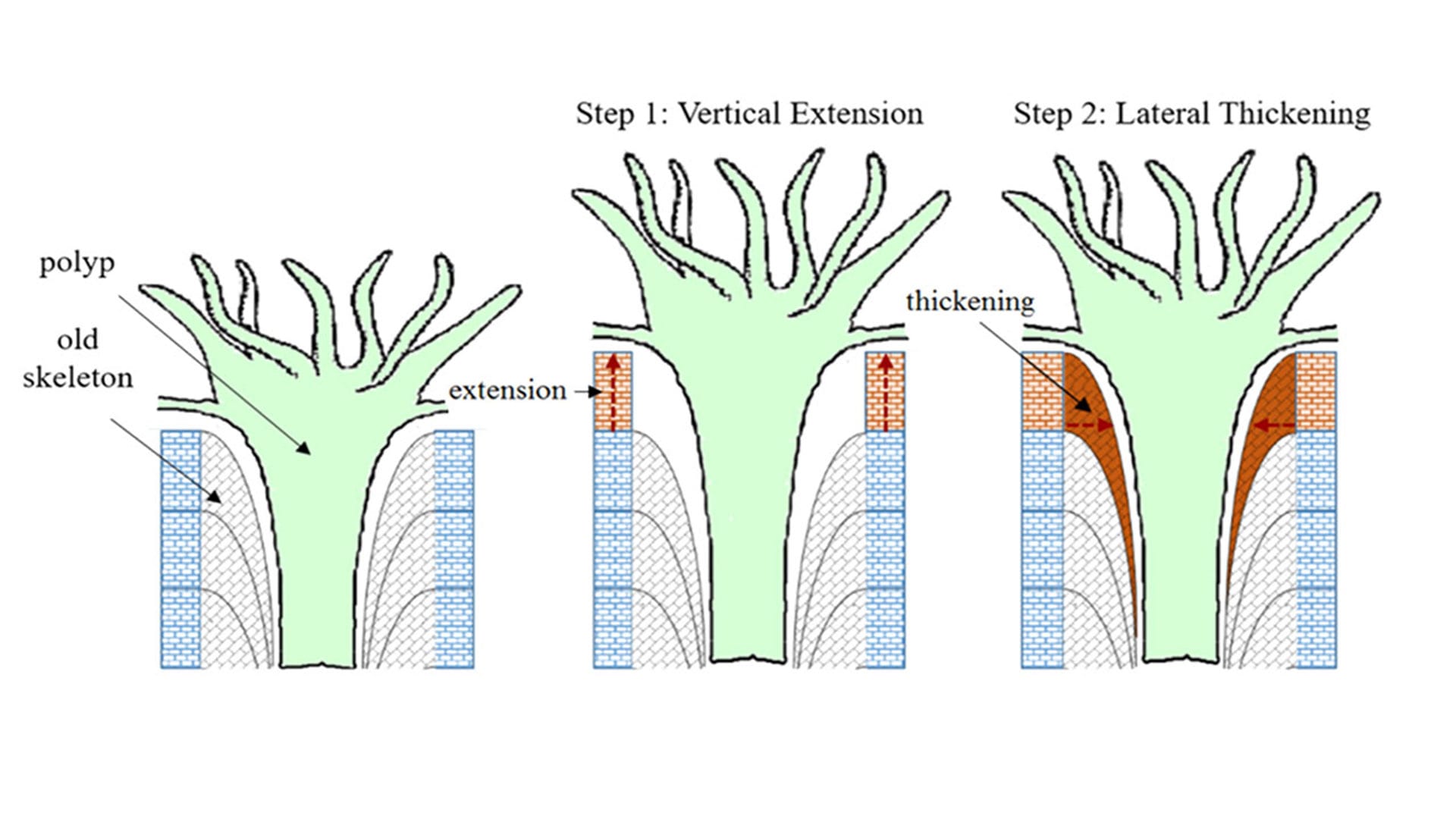
Coral polyps—the tiny living soft-bodied coral animals—grow up toward sunlight by constructing a framework of aragonite crystals. At the same time, they buttress this framework with bundles of additional crystals, which thicken and strengthen the skeletons to help them withstand breakage caused by currents, waves, storms, and boring and biting by worms, molluscs, and parrotfish. (Mollica et al., Proceedings of the National Academy of Sciences)
Coral skeletons are made of aragonite, a form of calcium carbonate. To grow up toward sunlight, corals construct a framework of aragonite crystals. At the same time, they buttress this framework with bundles of additional crystals, which thicken and strengthen the skeletons to help them withstand breakage caused by currents, waves, storms, and boring and biting by worms, molluscs, and parrotfish.
Ocean acidification is caused by rising levels of carbon dioxide in the atmosphere, mostly from burning fossil fuels. The carbon dioxide (CO2) is absorbed by seawater (H2O), setting in motion chemical reactions that produce more bicarbonate (HCO3-) and fewer carbonate (CO32-) ions. Coral polyps—the tiny living soft-bodied coral animals—bring in seawater containing these ions, along with calcium (Ca2+) ions, into a “calcifying space” between its cells and the surface of their existing skeletons. They pump hydrogen ions (H+) out of this space to produce more carbonate ions (CO32-) ions that bond with (Ca2+) ions to make calcium carbonate (CaCO3) for their skeletons. Because there are more HCO3- ions but fewer CO32- ions in acidified seawater, the corals have to expend more energy to pump out H+ ions from their calcifying space to build skeletons.

Woods Hole Oceanographic Institution graduate student Nathaniel Mollica (left) and WHOI scientist Weifu Guo examine a core extracted from a coral skeleton. They investigated how ocean acidification affects coral skeletal growth and identified where coral reefs may be more vulnerable in the future. (Anne Cohen Lab, Woods Hole Oceanographic Institution)
Laboratory experiments and field studies, however, have shown that acidification affects skeletal growth in some cases, but not in others. To explore this ambiguity, a research team led by Woods Hole Oceanographic Institution scientists Weifu Guo, Anne Cohen, and Mollica dove into the problem. Literally. Something Mollica had never envisioned he would be doing in his career.
A meandering career path
Mollica grew up in Fort Collins, Colo. “My mom was a ballet teacher and my dad ran a landscaping business,” he said. “My parents took me out hiking, and I always enjoyed nature. My dad had a degree in botany, and we’d go up and down trails and he would say, ‘Nathan, that plant is called this and, that one is called that,’ and would make me repeat it back to him.”
“Despite my interest in the outdoors, I was never really interested in science until middle school, when I was close to failing biology because I wasn’t turning in my homework,” he said. “My teacher offered me extra credit if I participated in an academic competition called Science Olympiad. I said, I would, because I thought it would get me out of a tight spot with my parents.”
“But it was great,” he said. “I continued those competitions throughout high school and met a lot of people interested in science.” To prepare to answer questions for the competitions, he pored through science textbooks that his coach sent him home with. His high school team won regional, then state, competitions, and went to the national Science Olympiad every year. He also competed in a Jeopardy-style competition called the Science Bowl. “It was a lot of fun.”
His favorite subject was geology, which led him to apply to the Colorado School of Mines. The school was primed to train students to go into applied fields in the oil and gas industry or civil engineering. Those didn’t interest Mollica, so he took a year off, taught math at a refugee school in Denver, and looked at options for graduate school.

A thriving coral reef community at Hotsarihie, Republic of Palau. A research team led by Woods Hole Oceanographic Institution identified a detailed mechanism showing how ocean acidification affects coral skeletons—giving scientists a way to predict more precisely where corals will be more vulnerable. The study was published Jan. 29, 2018, in the Proceedings of the National Academy of Sciences. (Hannah Barkley, Woods Hole Oceanographic Institution)
His particular interest was in the formation process of carbonate rocks, and his grad school explorations led to Cohen, a scientist in WHOI’s Geology and Geophysics Department. Cohen informed him that her lab didn’t do research on carbonates in a geological sense but on the primary architect of carbonate systems: corals. More specifically, the lab focused on how corals are affected by their environment, and how that, in turn, affects their ability to produce carbonate skeleton.
“That became way more interesting to me,” Mollica said. “Corals are the ultimate carbonate generator. Coral reefs have persisted through geological history and built most of the carbonate geology that there is. It was cool to be handed what I was looking for without knowing what I was looking for. I never thought I was going to be an oceanographer—right up until it happened.”
Diving into his research
Among the first things Mollica had to do when he arrived at WHOI was take a month-long course to learn how to scuba dive for his research. He and colleagues dove on reefs and used a drill to extract tubular, 3.5-centimeter-diameter cores from coral skeletons in four locations in the Pacific Ocean, where seawater conditions spanned a wide range of pH levels and carbonate ion concentrations.
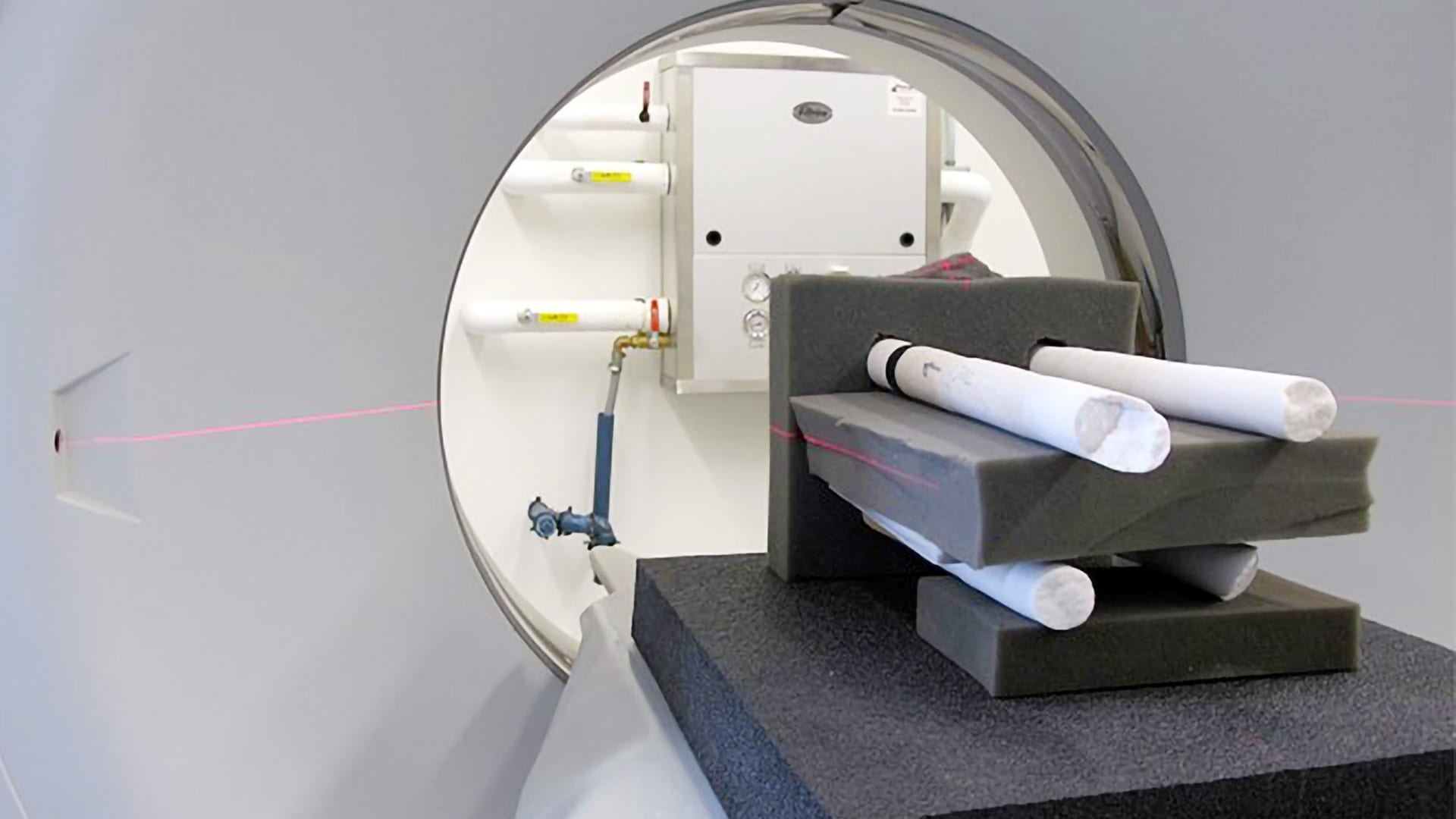
Scientists used a 3-D Computerized Tomography (CT) scanner to image the coral cores. The CT scan images reveal annual growth bands, much like rings on a tree, which showed scientists how corals grew their skeletons upward while also thickening them. (Tom DeCarlo, Woods Hole Oceanographic Institution)
The researcher team, which also included Cohen and WHOI scientist Weifu Guo, used a 3-D Computerized Tomography (CT) scanner to image the skeletal cores, which reveal annual growth bands, much like rings on a tree. From the scans, they could discern the upward and thickening components of the coral growth. Their analyses revealed that skeletons of corals in more acidic (lower pH and fewer carbonate ions) waters were significantly thinner. But they found no correlation between upward growth and carbonate ion concentration.
The researchers examined the coral growth process more closely. They determined that declining pH and carbonate ions in seawater strongly affected the corals’ ability to produce the aragonite bundles it uses to thicken their skeletons. In acidified conditions, corals continue to invest in upward growth, but “densification” or thickening suffers. As a result, the less-dense skeletons of corals in lower pH waters are more susceptible to damage from pounding waves or attacks by eroding organisms.

A 3-D CT scan reconstruction of a core sample of a coral skeleton. (Anne Cohen Lab, Woods Hole Oceanographic Institution)
Finally, Mollica and the research team developed a model simulating this new-found detailed skeletal growth mechanism and coupled it with projected changes in ocean pH around the world. They published their results in January 2018, in the journal Proceedings of the National Academy of Sciences.
“By incorporating the nuances of coral skeletal growth,” Mollica said, “we can more precise project how, where, and by how much, ocean acidification will affect tropical reef-building corals.”
The research team included Nathan Mollica, Weifu Guo, Anne Cohen, and Andrew Solow (WHOI), Kuo-Fang Huang (Academia Sinica in Taiwan), and Hannah Donald and Gavin Foster (University of Southampton in England). The research was funded by the National Science Foundation, The Robertson Foundation, the WHOI Ocean Life Institute, and the WHOI Investment in Science Fund.




