FAQ: Radiation from Fukushima
March 11, 2011
On March 11, 2011, a magnitude 9.0 earthquake—one of the largest ever recorded—occurred 80 miles off the coast of Japan. The earthquake created a series of tsunamis, the largest estimated to be over 100 feet, that swept ashore. In addition to the tragic toll of dead, injured, and displaced people, the earthquake and tsunamis badly damaged the Fukushima Dai-ichi nuclear power plant, eventually causing four of the six reactors there to release radiation into the atmosphere and ocean.
I began my career in oceanography by studying the spread of radionuclides from Chernobyl in the Black Sea. Since mid-2011, I have worked with Japanese colleagues and scientists around the world to understand the scope and impact of events that continue to unfold today. In June 2011, I organized the first comprehensive, international expedition to study the spread of radionuclides from Fukushima into the Pacific, and I or members of my lab have participated in several other cruises and analyzed nearly one thousand samples of water, as well as dozens of samples of sediment and biota. In the months after Fukushima, I also formed the Center for Marine and Environmental Radioactivity, in part to help share the most accurate, up-to-date information about radiation from human and natural sources and, when it became clear that there was no coherent and consistent source of government funding to monitor radiation in U.S. waters and to support public education, I formed a citizen-science/crowd-funding initiative called Our Radioactive Ocean at WHOI. These are a few of the most common questions that people have been asking me.
-Ken Buesseler, Woods Hole Oceanographic Institution
What has been released from the Fukushima reactors and how dangerous is it?
Releases from the Fukushima reactors have included dozens of radioactive elements, but with regard to materials released into the ocean, most of the attention has been on three radioactive isotopes released in large amounts: iodine-131, cesium-137, and cesium-134. Iodine-131 decays quickly and any that was released from Fukushima is no longer detectable in the environment, but it was a significant health concern at the start of accident. Cesium-137 and -134 were released in the largest amounts. At the height of the accident, levels in the ocean near the docks at the reactors were 50 million times higher than before the accident and, at those levels, were a direct threat to marine life. Levels dropped quickly after the first month and today are many thousands of times lower, which is less of a direct health threat, but still an indication of ongoing leaks.
Cesium-137 has a relatively long half-life (30 years), but it is also present in the ocean as a result of nuclear weapons testing in 1950s and 1960s. Cesium-134 is much shorter-lived, which means that any detected in seawater samples must have come from Fukushima. Because it was released in equal amounts with cesium-137, we can use its presence to determine how much contamination was released from the reactor site.
» More about iodine-131 and cesium-137
How will the radioactive material released in Japan affect humans?
Every additional source of radioactivity carries some additional health risk, but these risks vary with many factors, including the dose (how much a person is exposed to and for how long) and which isotopes you are exposed to, as well as individual sensitivities—there is a higher concern in children, for example. Fukushima will likely have the most significant long-term health impacts on those who had the highest exposures, so those living closest to the plant or in areas with higher fallout. This is because the further the radioactive material travels, the more dispersed (and the less harmful) it becomes.
Although measuring levels of radioactive contaminants in the oceans is challenging, measuring health effects associated with those levels is even more difficult and controversial. This is in part because increases in cancer are hard to attribute to any single cause and it is difficult to detect small increases in cancer over time when 30 percent of us will get cancer of some form in our lifetimes. We should always be concerned, but we should also realize that different levels, time, and manner of exposure can have widely varying health risks.
» More about the human health risks of radiation
Are the continued sources of radiation from the nuclear power plants of concern?
The site of the Fukushima Dai-ichi nuclear power plant is an ongoing source of radionuclides (pdf) in to the ocean—something I’ve seen evidence of in my data and published since 2011. However, the rate of release has fallen significantly since March 2011. At current rates of release, it would take 5,000 years to equal the amount of cesium that entered the ocean in the first month of the accident. For the workers at the site, direct exposure from leaking storage tanks is of greater health concern because exposure from these concentrated sources is much higher. For the general public, it is not direct exposure, but uptake by the food web and consumption of contaminated fish that is the main health concern from the oceans.
How long will radiation from Fukushima remain in the environment?
Radioactive materials are, by their very nature, unstable and decline in concentration over time. This change is measured in half-lives—the length of time it takes for the radiation to decrease by one-half. Every radioactive substance has a different half-life, ranging from fractions of a second to billions of years. Cesium-137, for example, has a half-life of 30 years and so, depending on its concentration, is a potentially serious health threat for decades or centuries. Iodine-131, on the other hand, has a half-life of just 8 days and so loses much of its potency after just days and effectively disappears after one to two months.
Another radionuclide of concern, cesium-134, has a half-life of two years, which means that it is rapidly disappearing. Because of its short half-life, cesium-134 is the one isotope that, if we find it, could have come only from Fukushima. It was also released in equal proportion with cesium-137, so when we detect cesium-134, we first adjust for its decay over time and then we can calculate how much total contamination was released. But this “fingerprint” provided by cesium-134 is rapidly fading.
Strontium-90 has a half-life of 29 years—nearly identical to that of cesium-137, but initially there was much less strontium than cesium released, and we found 40 times less strontium-90 than cesium-137 in the ocean in 2011. On land, however, there is over 1,000 times less strontium-90 than cesium-137. Over time, my lab and our colleagues in Spain, Switzerland, and Australia will continue to monitor strontium-90, tritium and several other isotopes in the ocean, seafloor and marine biota.
» More about half-lives
What are you testing for?
To date, we have focused our efforts on testing for the two isotopes of cesium (137 and 134) because they provide the first indication of whether contamination from Fukushima is present in a sample. We would generally not be able to measure strontium or plutonium or other radioactive elements if we could not detect cesium because these other elements would be present in such minute amounts that our instruments are not capable of detecting them.
Moreover, processing samples for strontium is very labor-intensive, requiring at least a full day of lab work as well as several days on the detectors. We did re-analyze some of our samples from the West Coast that contained Fukushima cesium-134, and have not detected any additional strontium-90. This new result makes sense, as the amount of strontium measured in the ocean near Fukushima was 40 times lower than cesium. This supports our idea that contaminated waters in the western Pacific today originated from near Japan 5 years ago.
Plutonium was also released from Fukushima, but in concentrations even lower than strontium. At the moment, concentrations of plutonium in waters off Fukushima are so low that background radiation from nuclear weapons testing more than 50 years ago makes the signal undetectable with our instruments. Japanese scientists using extremely sophisticated equipment have documented plutonium on land near the reactors at levels one million times lower than cesium in the same sample.
» More about what the Japanese scientists found here and here.
Where does radiation from Fukushima go once it enters the ocean?
The spread of cesium once it enters the ocean can be understood by the analogy of mixing cream into coffee. At first, they are separate and distinguishable, but just as we start to stir, the cream forms long, narrow filaments or streaks in the water. In the Pacific, streaks of contaminants become longer and narrower as they move offshore, where diffusive processes begin to homogenize and dilute the radionuclides. Currents then mix and continue to dilute the cesium as it travels across the ocean and, with distance and time, radionuclide concentrations in seawater decline.
» More information about our oceanographic studies off Fukushima, the movement of radiation across the Pacific (pdf), and radiation in sediments near Japan.
Is radiation a concern along U.S. and Canadian coasts?
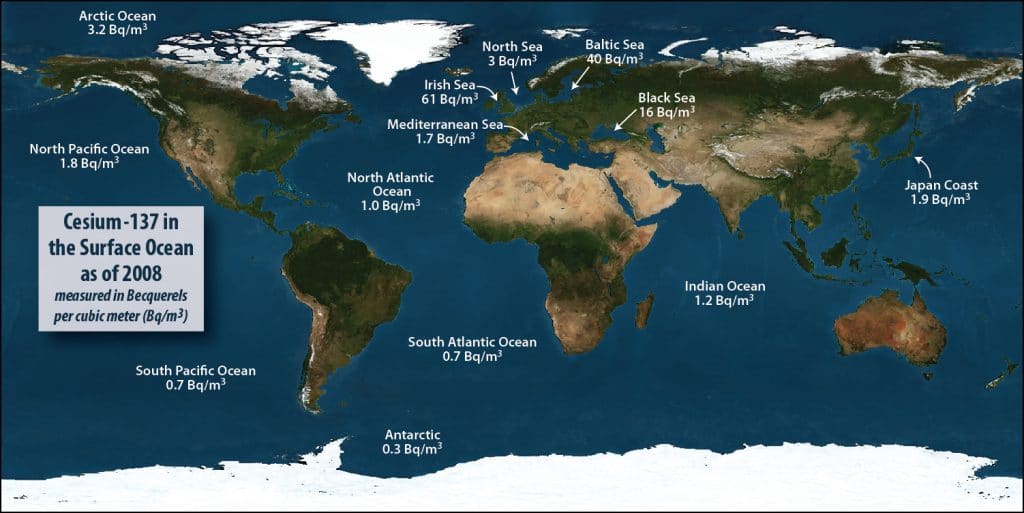
Although we have found traces of radioactive contamination from Fukushima in samples collected through our citizen-science initiative Our Radioactive Ocean, the concentration of cesium-137 and -134 in these samples is well below levels of concern for humans or marine life. The highest levels of cesium (10 Bq/m3) attributable to Fukushima that we have measured were found 1,500 miles north of Hawaii. Swimming every day in the ocean there would still result in a dose 1,000 time smaller than the radiation we receive with a single dental x-ray. Not zero, but still very low.
Looking ahead, levels of any Fukushima contaminants along the West Coast of North America are predicted to peak around 2015 or 2016, but at levels similar to what we are measuring in some of our samples today. This is not to say that we should not be concerned about additional sources of radioactivity in the ocean above the natural sources, but at the levels expected, even short distances from Japan, the Pacific will be safe for boating, swimming, etc. Nevertheless, we continue to monitor levels of radiation up and down the West Coast through Our Radioactive Ocean.
» More about what we have found off the West Coast here and here.
Has Fukushima been responsible for the deaths of marine animals in the Pacific?
To date, there have been no reliable links made between radiation in the Pacific and mass die-offs of marine mammals, birds, fish, or invertebrates. Some of these die-offs have been attributed to viruses, warming water, and other changes to the marine environment that need to be addressed. If there were effects from radioactive contamination, we would expect to see the largest effects off Japan, not the West Coast of North America, and this has not been seen.
» More about the history of mass mortality events in the environment
How far can radiation travel?
Ionizing radiation itself cannot travel very far through the air. Typically, dust and other particles, seawater and other liquids, or even gases pick up radioactive contaminants that are then transported great distances. In the months and years after the explosion at the Chernobyl nuclear power plant in Ukraine, scientists were able to track the spread of radioactive material in the atmosphere and the ocean around the globe. Within a week after the explosions at the Fukushima plant, there were reports of very small increases in radionuclides on the continental U.S.
» More about mapping and monitoring radiation from Japan and the different types of radiation.
If radioactivity from Fukushima was released into the atmosphere, should I be worried when flying?
Immediately following the nuclear accident at Fukushima, radioisotopes were released into the atmosphere and were quickly carried around the globe by the wind. Gases and small aerosol particles (dust) are the main carriers of the radioactive materials. We detected extremely low levels in the atmosphere here on Cape Cod 10 days after the first releases, despite the distance from Fukushima. Iodine was the main isotope detected but it has a very short half-life (8 days) so it disappeared very quickly. The only population of concern would be those in close proximity to the accident and fortunately the wind blew most of this contamination offshore.
These radioactive elements are generally carried by dust and fall quickly out of the atmosphere near the source via rain and settling, and are not a concern for flying in airplanes. When you fly in an airplane you are exposed to natural sources of radiation from cosmic rays emitted by the sun. Exposure to these additional cosmic rays is not detectable in the health of pilots or those who spend a great deal of time flying.
Is radiation exposure from the ocean and beach a concern?
I stood on the deck of a ship l2 miles from the Fukushima reactors in June 2011 and was about one-half mile away as recently as October 2015 and the radiation detectors I was carrying showed little or no increase above background levels. Even the samples I collected (water, sediment, plants, and animals) from these locations are safe to handle without any precautions. In fact, our biggest problem is blocking interference from background radiation in our samples so we can isolate the trace levels of cesium and other radionuclides that we know came from Fukushima.
On the West Coast of North America, radiation from the water, sediment, and biota is even less of a problem because of the distance from Japan and the dilution that occurs as the contaminants cross the Pacific. The greatest concern is for those who work on the site of the reactors because leaks from storage tanks could release water with high concentrations of contaminants.
How does Our Radioactive Ocean measure radiation in seawater samples?
We use a method that is capable of detecting extremely low levels of the specific radioactivity produced by cesium isotopes released from Fukushima in seawater. First we pass a seawater sample through a column of cesium-absorbing beads made of a resin that has been optimized for use with seawater. Then we dry the resin and place it in a high-purity germanium well detector made by Canberra Industries for between 24 and 72 hours.
Every time a cesium atom decays, that event is registered in the instrument’s detector, which has the ability to discern energy given off by two critical isotopes of cesium: cesium-134 and cesium-137. By counting the decay events associated with each isotope, we can calculate the total counts per second (cps) for a given sample. Knowing the efficiency of our detectors and something about the decay properties of the isotopes allows us to calculate the concentration of both cesium isotopes in a sample. This number is often reported in activity units of Becquerels per cubic meter (Bq/m3), where one Bq equals one decay event per second and one cubic meter equals 1,000 liters (about 264 gallons) of seawater.
We regularly participate in proficiency tests with the International Atomic Energy Agency (IAEA) to ensure that our results are not just precise, but extremely accurate when compared to international seawater standards. In general, larger sample sizes (we process a relatively large 20 liter sample), longer counting times (we typically leave a sample on for 48 hours or more), and more efficient detectors (we use some of the world’s most sensitive gamma detectors) lead to the lowest possible detection limits.
I have a Geiger counter. Can I use it to detect radiation from Fukushima?
There are two basic types of radiation detectors—those that measure only the number of times radiation interacts with the instrument, and those that measure the energy level (in electron volts) of the particles or waves detected by the instrument. The Geiger-Mueller tube (Geiger counter) is perhaps the most widely known radiation detector and falls into the first category.
Geiger counters can measure beta particles and gamma rays (the detector window will block most alpha particles), but cannot distinguish between the two. These interactions, and the decay events that trigger them, are registered as counts or audible clicks. In general, a Geiger counter will always produce some clicks, often 20 to 40 per minute, as a result of natural sources of radioactivity around us at all times, including rocks, soil, buildings and cosmic particles. These background count rates vary widely depending upon local geology, altitude (higher at higher elevations), and even construction materials and building design (the accumulation of radon in basements is just one example). Detecting contamination from Japan above this background with a Geiger counter is only possible near the reactors and storage tanks at Fukushima, or in some of the more contaminated regions in Japan, as they are not particularly sensitive instruments.
In addition, Geiger counters cannot measure the energy level of the radiation being emitted, a very important factor in determining whether the source of radiation is manmade or natural. For example, the high count rates detected by a Geiger counter along a beach near San Francisco were not caused by cesium from Fukushima as originally reported, but rather caused by naturally occurring thorium-bearing minerals that are common and often elevated in some beach sands.
» More about measuring radiation.
Are there other ways to detect Fukushima radiation in the ocean?
In addition to measuring bulk seawater samples, as we do, other labs have analyzed radiation in fish and kelp. The studies provide much-needed information that seawater samples do not, but also present some issues of their own. Analyzing fish and other seafood, for example, tells us how much radiation a person or other marine animal might be exposed to by eating the contaminated organism, but it does not tell us how far the plume has spread from Fukushima or the concentration of the various radionuclides in the water where the organism was exposed.
Studies of kelp provide integrated time averaged, qualitative measure of kelp exposure to a wide range of radionuclides in the ocean, but do not give a precise indication of the exact level of the radionuclides at a given point in time in the ocean, as levels in kelp will vary not just with water concentration changes during the kelp growth cycle, but also variables such as ocean currents, and kelp physiology. As a result, direct collection and analysis of radionuclides in water samples is the best way to determine how much contamination is in the ocean that poses an exposure risk to people and marine life.
» More about Fukushima radiation in the ocean.
Are there different types of radiation?
In general, there are two types of radiation, ionizing and non-ionizing. Non-ionizing radiation includes visible light and radio waves—things that, as the name implies, do not have the ability to form charged ions in other materials. Ionizing radiation, however, does form charged ions and as a result presents a serious health threat because it can alter the atomic structure of living tissue. Ionizing radiation also comes in several different types, including alpha, beta, and gamma radiation, all with different degrees of concern and health impacts.
» More about types of radiation
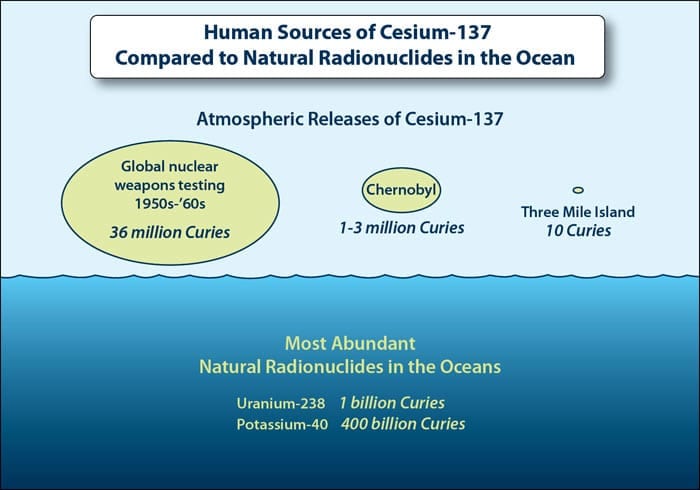
What is the normal background level of radiation?
The normal background level of radiation is different for different places on the planet. Radiation in some places is higher because these receive less of the natural protection offered by Earth’s atmosphere or because they are in places where the surrounding rocks contain more radioactive substances, such as radon. In the ocean, the largest source of radiation comes from naturally occurring substances such as potassium-40 and uranium-238, which are found at levels 1,000 to 10,000 times higher than any sources of radiation caused by humans. The largest human release of radionuclides was the result of atmospheric nuclear weapons tests carried out by the U.S., France, and U.K. during the 1950s and 60s. Despite even the high concentration of nuclear fallout in the Pacific caused by U.S. tests on the Marshall Islands, there is no measurable health effect that would prevent us from eating seafood from the Pacific.
» More about natural background radiation (pdf)
What is the state of fisheries off Japan and along U.S. West Coast?
Most Japanese fisheries were unaffected by Fukushima, but coastal fisheries nearest the reactors remain closed because of concern over exposure by some species, particularly those that live on or near the seafloor. These are being tested on a regular basis against Japan’s limits for radiation in seafood (which are much more strict than U.S. regulations) and these contaminated fish are not being sold internally in Japan, nor are they being exported. There is currently no concern about the levels of cesium and other radionuclides in fish off the West Coast of the U.S., nor have there been at any time since 2011.
» More about the state of Japanese fisheries (pdf) and monitoring of biota in the Pacific.
Are fish such as tuna that might have been exposed to radiation from Fukushima safe to eat?
Seawater everywhere contains many naturally occurring radionuclides, the most common being polonium-210. As a result, fish caught in the Pacific and elsewhere already have measurable, but small, quantities of these substances. Most fish do not migrate far from their spawning grounds, which is why some fisheries off Fukushima remain closed. But some species, such as the Pacific bluefin tuna, swim long distances and could pick up cesium in their feeding grounds off Japan before crossing the Pacific.
However, cesium is a salt like potassium, and it will begin to flush out of exposed fish soon after they enter waters with lower contamination from Fukushima. By the time tuna are caught in the eastern Pacific, cesium levels in their flesh are 10-20 times lower than when they were off Fukushima. A study published in 2012 in the Proceedings of the National Academy of Sciences (PNAS) reported finding very low levels of cesium in Pacific bluefin tuna caught by recreational fisherman off the coast of California in August 2011. The FDA reviewed this study and determined that the levels of cesium were roughly 300 times lower than levels that would prompt FDA to investigate further to determine if there were a health concern.
» More about the risk of consuming seafood from the Pacific here (pdf) and here.
Is there concern about other radionuclides, such as strontium-90?
The continued release of radionuclides from groundwater and leaking tanks at Fukushima nuclear power plants site needs to be watched closely, as the character or mix of radionuclides is changing. One example is the higher levels of strontium-90 contained in groundwater and in storage tanks that are leaking into the ocean. Because strontium-90 mimics calcium in humans and animals, it is taken up by and concentrated in bones, where it remains for long periods of time (it has a half-life of 29 years and it is is not replaced as quickly in the body as cesium).
What we see is that the levels of cesium in the ocean are decreasing faster than strontium near the Fukushima nuclear power plant site. However, levels of both are much lower than at their peak in 2011. We remain most concerned about the potential of new releases from the thousands of storage tanks on the site, which contain highly radioactive water awaiting processing. Some leaks have been reported, and one reason we continue to monitor strontium is to look for signs of these leaks. Given that strontium concentrates in bones, this radionuclide could become a larger concern in small fish such as sardines, which are often eaten whole. So far, however, evidence suggests that levels of strontium-90 in fish remain much lower than those of cesium-137.
Is it safe to eat seafood from the Pacific?
Except for the vicinity of the reactors, seafood and other products taken from the Pacific should be safe for human consumption. Radiation levels in seafood should continue to be monitored, of course, but radiation in the ocean will very quickly become diluted and is not of concern by the strict standards used in Japan beyond the region closest to Fukushima. The same is true of radiation carried by winds around the globe. However, crops and other vegetation near the reactor site (including grass that cows eat to produce milk) that receive fallout from the atmosphere build up radioactivity and can remain contaminated even if washed. When these foods are consumed, a person receives much of this dose internally, often a more severe pathway to receive radiation than by external exposure.
» More about radiation and food safety
Is there an easy way for me to test fish or water at home?
Unfortunately there is no simple way to test fish or other seafood at home for radiation contamination. The levels found in most animals are far too low to be detected by a Geiger counter or other readily available detector. As for water, other than funding and sending us a sample to analyze there are no simple ways to test your home drinking water for cesium. We use 20 liter (5 gallons) samples that we filter through a special resin that cesium attaches to. We then place this concentrated sample on an extremely sensitive detector for a day or more to measure the amount of cesium-137 and -134 that it contains.
» More about our testing methods.
Is debris washing ashore on the US/Canadian West Coast of concern?
Over one million tons of debris washed out to sea by the tsunami drifted across the Pacific but did not carry Fukushima radioactive contamination (I’ve measured several samples in my lab). This is in part because it entered the ocean days before the major radioactive releases began, and many of the most abundant radioactive contaminants do not concentrate on wood, plastics and other floating materials. It did, however, carry invasive species, which are of concern to coastal ecosystems on the West Coast.
How does radiation released from the Japanese reactors compare to the accident at Chernobyl?
The Chernobyl accident released higher levels of radioactivity, but this varies depending upon which radioactive contaminants you are talking about. The difference is because Chernobyl was a much more violent event that included a large explosion resulting in a complete breach of the reactor vessel. The event also started a very hot graphite fire that released large amounts of radioactive material into the atmosphere equivalent to between 3 and 5 percent of the total reactor inventory. Winds carried the radioactive fallout first to the north and eventually into the Black Sea to the south. Radiation in the Black Sea and Baltic Sea, though elevated, remained well below what was seen in the ocean off Fukushima, because Chernobyl is so much further from the ocean.
Although Fukushima included explosive events attributed to the escape and ignition of hydrogen gas, the main reactor vessels were not breached to the extent that occurred in Chernobyl. As a result, releases from Fukushima consisted primarily of gases and those contaminants that, under high temperature, become gases. These so-called “volatile” elements includes cesium, but not strontium or plutonium, which is why there were much smaller release of these non-volatile contaminants from Fukushima than from Chernobyl.
» More about the after-effects of Chernobyl and a comparison of Chernobyl and Fukushima.
Why is the Fukushima accident of interest to oceanographers?
In addition to measuring the concentration and spread of radioactivity in the ocean, scientists can also use these radioactive contaminants to learn about ocean properties and processes. Oceanographers use substances called tracers to study the path and rate of ocean currents and of processes such as mixing that are important parts of the global ocean and climate systems. There are many different radionuclides that scientists use as “clocks” to measure how fast the ocean mixes different water masses and sediment accumulates on the seafloor. Some of these substances are natural, but many are the result of human activity, such as the Chernobyl accident or nuclear weapons testing, and now releases at Fukushima.
» More about radioactive tracers in the ocean
Updated March 2016