Study Reveals Microbes Dine on Thousands of Compounds in Oil
September 30, 2008
Thousands of feet below the bottom of the sea, off the shores of Santa Barbara, CA, single-celled organisms are busy feasting on oil.
Until now, nobody knew how many oily compounds were being devoured by the microscopic creatures, but new research led by David Valentine of University of California at Santa Barbara (UCSB) and Chris Reddy of Woods Hole Oceanographic Institution (WHOI) in Massachusetts has shed new light on just how extensive their diet can be.
In a report published in the Oct. 1 edition of the journal Environmental Science & Technology, researchers — Valentine, Reddy, lead author George Wardlaw, a graduate student in the Marine Science program at UCSB, and three other co-authors from Swiss Federal Institute of Technology and WHOI — detail how the microbes are dining on thousands of compounds that make up the oil seeping from the sea floor.
“It takes a special organism to live half a mile deep in the Earth and eat oil for a living,” said Valentine, an associate professor of earth science at UCSB. “There’s this incredibly complex diet for organisms down there eating the oil.”
And, the researchers found, there may be one other byproduct being produced by all of this munching on oil — natural gas. “They’re eating the oil, and probably making natural gas out of it,” said Valentine. “It’s actually a whole consortium of organisms — some that are eating the oil and producing intermediate products, and then those intermediate products are converted by another group to natural gas.”
Reddy, a marine chemist at WHOI, said the research provides important new clues in the study of petroleum. “The biggest surprise was that microbes living without oxygen could eat so many compounds that compose crude oil,” Reddy said. “Prior to this study, only a handful of compounds were shown, mostly in laboratory studies, to be degraded anaerobically. This is a major leap forward in understanding petroleum geochemistry and microbiology.”
The diet of the single-cell microbes is far more diverse than previously thought, Valentine said. “They ate around 1,000 of the 1,500 compounds we could trace, and presumably are eating many more,” he said.
Research for this project began several years ago when Valentine brought Reddy out to the natural hydrocarbon seep field right off the California coast. Approximately 100 barrels of oil ooze out of the sea floor there each day and bubble up 15 meters (45 ft) to the sea surface. When the oil reaches the sea surface it is chemically changed by evaporation and sunlight, the action of the water, and bacterial activity.
“I was blown away by what I saw,” said Reddy, who has undertaken a number of long-term studies on the impact of oil spills on the environment. “Normally I arrive at an oil spill a day or two after it has happened, and the most toxic, short-lived compounds have already disappeared. I knew right away this site provided an important window into the fate of oil in the environment, by providing the opportunity to simultaneously sample oil from its sub-seafloor source to the sea floor and up to the surface. It’s a really powerful snapshot.”
Natural seeps contribute nearly half of the oil entering the coastal ocean. However, environmental fate studies generally monitor fewer than five percent of these petroleum compounds. Using a new technique devised by Reddy, the scientists were able to pick apart the differences in the makeup of the oil along its migration route to the surface through faults from deep below the sea floor. The microbes prefer the lighter compounds of oil — the gasoline part of the black goo. They tend to leave behind the heavily weathered residue, which is what makes its way to the surface and, sometimes, to the beaches in the form of tar.
“There always seems to be a residue,” Valentine said. “If you think of oil as a buffet for microbes, there’s a point where they (bacteria) hit a wall. There seems to be stages in which they eat. There’s the easy stuff — the steak. And then they work their way to the vegetables, and then garnish, and then they stop eating after awhile. It just depends on how hungry they are and what’s fed to them.”
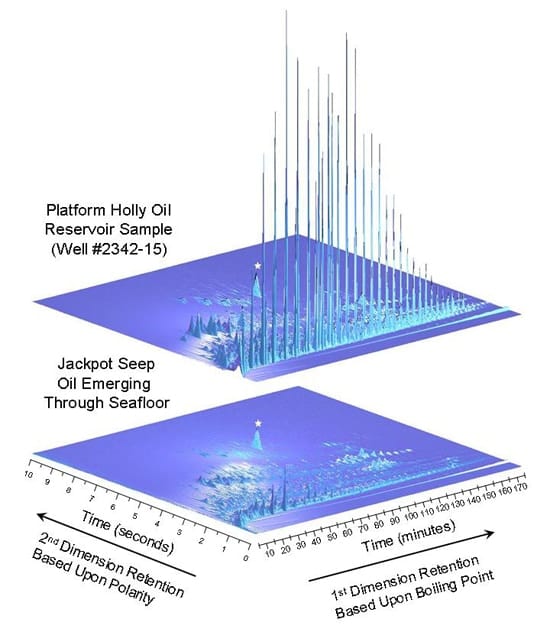
The samples were sent to Reddy’s lab in Woods Hole to be analyzed using a diagnostic technology called a comprehensive two-dimensional gas chromatography (GCxGC). Typically, chromatography involves heating up a sample and putting it into a column – a wrapped coil tube a little thicker than a strand of hair and approximately 60 meters long. Compounds are then separated based on their boiling points, which works well with light crude oil, Valentine said. But with the two-dimensional test, the compounds are cooled to stop their motion for about 10 seconds, and a flash pulse of hot air releases them into a second column, where the compounds travel at different speeds, allowing the researchers to differentiate the many thousands of compounds.
“This new technology was actually too good at its job,” Reddy said. “It was able to separate and help identify significantly more compounds in the oil samples than traditional analytical techniques. The result was that we were handcuffed with too much data afforded by the GCxGC. However, we overcame this hurdle by using new algorithms to help us interpret the data, which in turn led us to these milestone discoveries.”
The next steps in their research are already under way, according to Valentine. They are following the oil diet in controlled laboratory conditions, and tracking the fate of the oil once it forms a slick at the sea surface.
“When you fly out of the Santa Barbara Airport, you can look down and see these massive slicks,” Valentine said. “You can follow them for about 20 miles. A lot of the oil comes up on the beaches, but then what happens to it after that? Certainly the microorganisms continue to act on it. Evaporation occurs, but most of it can’t evaporate. Some of it breaks down from sunlight. So where does the rest of it end up? We want to know how far the organisms will go in eating the oil and what happens to the residual tar. It doesn’t all stick to our feet and there must be a lot of it out there somewhere.”
“The more biodegraded oil is, the ‘heavier’ it is and the less value it has,” Reddy added. “Our understanding of what these microbes eat is giving us a better grasp of the link between bacterial activity and the quality of oil.”
This work was funded by grants from the U.S. National Science Foundation, the U.S. Minerals Management Service, the California Toxic Substance Research and Training Program, the U.S. Department of Energy, and the Seaver Institute.
The Woods Hole Oceanographic Institution is a private, independent organization in Falmouth, Mass., dedicated to marine research, engineering, and higher education. Established in 1930 on a recommendation from the National Academy of Sciences, its primary mission is to understand the oceans and their interaction with the Earth as a whole, and to communicate a basic understanding of the oceans’ role in the changing global environment.