Jet Fuel from Algae?
Scientists probe fuel potential in common ocean plant
We believe we have found a recipe that could open a new door to producing jet fuel from ocean algae.
We are not claiming some Rumplestiltskinesque hay-into-gold finding—but rather a first step worth exploring. We took a closer look at a certain type of algae, which contain a class of fascinating and overlooked chemical compounds that most algae do not have. In a study published Jan. 26, 2015, in the American Chemical Society’s journal Energy & Fuels, we reported on a process to transform these compounds into components used in jet fuel.
Algae have been used before to make jet fuel and, more commonly, to make another type of fuel, biodiesel. But we have found a way to make both fuels, in parallel, from a single algae.
Think of us as two bakers who took a weed and made good batches of two different types of flour out of it; there’s still a lot of scaling up to do before we might bake millions of loaves of bread to feed the world. But, if you think about it, it’s not an unreasonable stretch because producing oil from algae is not new. Nature has been doing it for millions of years.
In fact, all of the oil and natural gas we use for energy are remnants of algal debris that has been buried and heated below Earth’s surface, where it has been “cooked and squeezed” as we sometimes say, into fossil fuels.
In the past several decades, there has been great interest in growing algae in artificial pools, skipping a few steps and a few million years, and creating renewable “biofuels” in days to weeks. Sparked by the 1970s energy crisis, numerous programs to produce alternative fuels were launched. From 1978 to 1996, the U.S. Department of Energy sponsored the Aquatic Species Program, which was charged with finding ways to convert algae into transportation fuel, mainly biodiesel. But it is never easy trying to accelerate a process that nature takes her time with. The program generated research inroads into the problem, but it petered out when estimated costs to produce fuels proved too expensive.
Fats = fuels
Like all plants, algae (and indeed most living things) produce sugars and oils (carbohydrates and fats) to store energy, and then burn them to fuel their movements and metabolic activities. We extract these compounds from plants, often in the form of oils, and use them to produce energy. Cooking oils are made of triglycerides (a type of fat) extracted from all sorts of plants: sunflower, safflower, olive, and rapeseed (canola), for example. Another example is ethanol, which is produced from corn sugars and blended in with gasoline.
In the past decade, interest in algal-based fuels has bloomed. This is in part a response to the U.S. Environmental Protection Agency’s Renewable Fuels Standard program, which committed the United States to replacing 36 billion gallons of its transportation fuel with renewable fuel by 2022.
Algal biofuel production also has several positive attributes that, combined, have reignited the interest of researchers and entrepreneurs around the world. For one, unlike corn and other crops that people eat, algae wouldn’t affect food supplies and prices if it were diverted to make fuel. In addition, algae can be grown in high quantities per acre, on otherwise nonproductive, non-arable land, and in a wide variety of water sources (fresh, brackish, saline, and wastewater). Compared to burning fossil fuels, burning biofuels from algae results in a net reduction of heat-trapping carbon dioxide. And the production of algal biofuels can also yield valuable co-products, as we show in our new study.
A little hype has also attracted attention to algae-based biofuels. Proponents note that some algae produce far more fats and sugars than soybean and other land-based plants. By these numbers, they say, as little as 3 percent of U.S cropland dedicated to algae could produce biodiesel that could meet the nation’s transportation fuel needs. There’s a big assumption in those projections, however. The estimates are often based on knowing how fast and how fat algae grow in fishtank-size aquaria, with the results extrapolated to millions of acres. It remains untried and unknown whether algae can be grown economically at industrial scales as large as, say, Nebraska.
Beyond the chromatographic pale
For our research, we targeted algae called Isochrysis, which seemed like a good candidate for feedstock for fuel. It contains fair amounts of fats and grows well. It is already grown commercially for fish food, so many challenges associated with growing beyond fishtank-scale already have been achieved. Yet as far as we know, very little work has been done to explore its fuel potential.
There are at least two reasons for this. First, researchers screen hundreds of different types of algae for their biofuel potential. A former worker at a major chemical company once told us that researchers there had examined Isochrysis, but they passed on it because the oil extracted from it wasn’t a clear yellow liquid like soybean oil, but rather a near-black greasy gunk that looked relatively hard to work with.
A second reason is that biofuel prospectors typically have their eye on fatty acid methyl esters (FAMEs), compounds that make good biodiesel. The standard analytical tool used to find chemical compounds in substances is called gas chromatography. The technique takes a mixed bag of chemicals in algae and separates them into their constituent compounds, according to their different masses. The FAMEs that are produced from triglycerides generally contain anywhere from 14 to 20 carbons. Any FAMEs in algae separate out after 30 to 40 minutes. If you kept the gas chromatogram running for another half hour, no further compounds would show up. So biofuel prospectors stop there.
If you keep the instrument running on Isochrysis for another hour, however, another class of much heavier compounds starts to appear. These compounds are what makes oil from Isochrysis semi-solid at room temperature and deterred people from considering it as a fuel feedstock.
We weren’t deterred, however, because we’ve worked at Woods Hole Oceanographic Institution and knew what oceanographers know about Isochrysis. (See New Use for a Well-Known Algae.)
A common but unusual algae
Isochrysis is in a division of algae called haptophytes. There are a few different types of haptophytes, but they grow abundantly in the sunlit surface waters of the ocean. Unlike most other algal species, haptophytes make chemical compounds that have long intrigued oceanographers.
These compounds were first discovered in seafloor sediments from Walvis Ridge off West Africa in the 1970s. They are called alkenones, and they are composed of long chains with 37 to 39 carbons, linked in segments by carbon-to-carbon double bonds. Their structures make them formidable to decomposition, and so they sink to the seafloor and abide in sediments.
In the 1980s, scientists determined that alkenone-producing algae respond to different water temperatures by synthesizing more double bonds in their alkenones when temperatures are colder and fewer when they are warmer. By measuring ratios of double bonds in alkenones preserved in the sediments, oceanographers can reconstruct past sea-surface temperatures. (With some sophisticated techniques, they have also analyzed alkenones to determine how Earth’s aridity and atmospheric carbon dioxide levels varied in the past.)
As a result, these remarkable compounds are among the most extensively studied class of organic compounds in marine science. Even so, scientists still don’t know why haptophytes manufacture alkenones along with the more common fats. Alkenones are fats, however, and it is safe to assume they are used for energy storage.
Feedstocks and double bonds
Our oceanographic knowledge of alkenones made them attractive as a potential feedstock for the production of renewable chemicals and fuels. Our plan was to identify if we could make biodiesel from the triglycerides in Isochrysis and, in parallel, use the alkenones— the “other fat”—as another fuel, or coproduct. Coproducts are in fact cited as one of the key reasons for exploring algae as a source of biofuels in the U.S. Department of Energy’s 2010 “National Algal Biofuels Technology Roadmap.”
In particular, we focused on the possibility of producing jet fuel. Typical FAMEs are too long to be used as jet fuel, but serve as an excellent “drop-in” replacement for fossil diesel. Alkenones are even longer, but we targeted their double bonds as weak links that we could chemically cleave to cut the long chain into much smaller segments. Indeed, if we could, the alkenones offer lots of usable material to work with—the chemical equivalent of starting with 37-to-39-foot boards of wood, instead of the “14-to-20-foot boards” in triglycerides.
We used a chemical process called olefin metathesis, which earned its developers, Robert Grubbs and Richard Schrock, the Nobel Prize in Chemistry in 2005. In our newly published study, we showed that it selectively cleaves carbon-carbon double bonds of alkenones. The double bonds—and hence the cleaving—are ideally positioned in alkenones to produce fragments with shorter lengths similar to compounds used for fossil-based jet fuels.
So we have isolated alkenones as a product with biodiesel oils and can use these unusual compounds made by a common algae to produce jet fuel. But based on the current cost of Isochrysis sold by a handful of vendors for purpose of shellfish feed (about $400 per kilogram), the fuels we have produced would cost at least $10,000 per gallon.
With the incentive of this new-found potential for Isochrysis, can we find ways to grow it and harvest its compounds at industrial scales and cheaper costs? Can we simultaneously make additional co-products from Isochrysis—effectively to use every part of the buffalo as native-Americans did—to increase the overall value of algal biomass? Our research is merely a first step, but it’s an intriguing one.
This research was funded by the National Science Foundation, the Massachusetts Clean Energy Center, and Woods Hole Oceanographic Institution.
Slideshow

Slideshow
- Greg O'Neil is lead author of a new study that demonstrates a way to produce two types of fuel—biodiesel and jet fuel—from a single algal species. O'Neil, a chemist at Western Washington University, collaborated with Chris Reddy, a chemist at Woods Hole Oceaongraphic Institution. (Tom Kleindinst, Woods Hole Oceanographic Institution)
- WHOI chemist Chris Reddy collaborated with Greg O'Neil to investigate algal-based biofuels. O'Neil was a guest student in Reddy's lab in 2002. Now a chemistry professor at Western Washington University, O'Neil has been on sabbatical at WHOI in 2014-2015. (Tom Kleindinst, Woods Hole Oceanographic Institution)
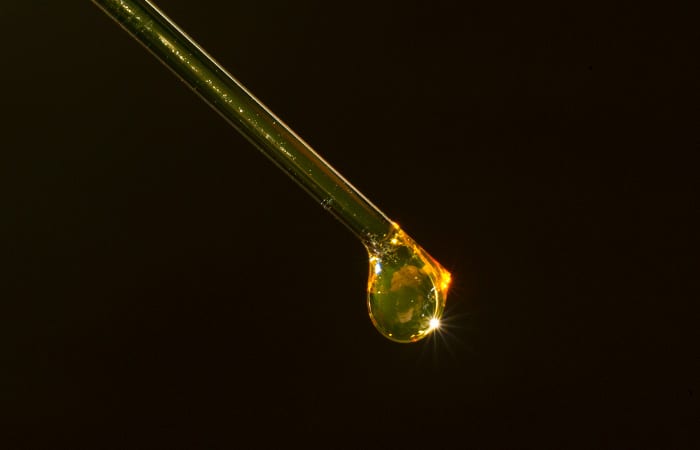
- Algae contain fatty acids that can be converted into fatty acid methyl esters, or FAMEs, the molecules in biodiesel. FAMEs produce clear liquids that look like cooking oils. (Tom Kleindinst, Woods Hole Oceanographic Institution)
- Biofuel prospectors may have dismissed the algal species Isochrysis as a good feedstock for biodiesel because its oil is a dark, sludgy solid at room temperature. That's because Isochrysis also contain large compounds called alkenones, which are solids at room temperatures and mix with the oils. But new research has shown a way to break alkenones into smaller compounds used in jet fuel. (Tom Kleindinst, Woods Hole Oceanographic Institution)
Related Articles
- Are warming Alaskan Arctic waters a new toxic algal hotspot?
- The Living Breathing Ocean
- Forecasting Where Ocean Life Thrives
- PlankZooka & SUPR-REMUS
- Illuminating an Unexplored Undersea Universe
- Specks in the Spectrometer
- Setting a Watchman for Harmful Algal Blooms
- Short-circuiting the Biological Pump
- A Telescope to Peer into the Vast Ocean
See Also
- Production of Jet Fuel Range Hydrocarbons as a Coproduct of Algal Biodiesel by Butenolysis of Long-Chain Alkenones Energy & Fuels
- 1 Algae 2 Fuels
- Greg O'Neil
- Chris Reddy
- Two fuels from a single algae WHOI news release
- New Use for Well-known Algae Oceanus magazine