Short-circuiting the Biological Pump
Tiny chemical compounds help choreograph a planet-size dance
The ocean has been sucking up the heat-trapping carbon dioxide (CO2) building up in our atmosphere—with a little help from tiny plankton. Like plants on land, these plankton convert CO2 into organic carbon via photosynthesis. But unlike land plants, decomposing plankton can sink into the deep ocean, carrying the carbon with them. It’s called the “biological pump,” and if it operated at 100-percent efficiency, nearly every atom of carbon drawn into the ocean would be converted to organic carbon, sink deep, and remain sequestered from the atmosphere for millennia. But like hail stones that melt before reaching the ground, some carbon never makes it to the deep ocean, allowing CO2 to remain at shallow depths and leak back into atmosphere. Graduate student Bethanie Edwards and colleagues discovered a surprising short-circuit to the biological pump. In a study published April 27, 2015, in the Proceedings of the National Academy of Sciences, they found that sinking particles of stressed and dying phytoplankton release chemicals that have a jolting, steroid-like effect on marine bacteria that feed on the particles. The chemicals juice up the bacteria’s metabolism causing them to more rapidly convert organic carbon in the particles back into CO2 before they can sink into the deep ocean. “We believe these compounds are acting as signals to the bacterial community to let them know phytoplankton are dying, that there’s lots of ‘free’ food on the way, and to ramp up their metabolisms,” said Edwards, who was lead author of the study, with co-authors Benjamin Van Mooy, her Ph.D. advisor at Woods Hole Oceanographic Institution (WHOI), and Kay Bidle from Rutgers University. “When the bacteria consume phytoplankton faster, more CO2 is given off in the shallow depths, where it can return to the surface of the ocean and to the atmosphere more quickly.”
A dinner bell for bacteria
Scientists investigating what controls the fate of carbon in the ocean have explored many factors, such as how easily phytoplankton particles break down, how much carbon they contain, and how fast they sink. Typically, the detritus of phytoplankton have no special effect on bacteria; they are simply a food source, said Edwards, a graduate student in the MIT/Woods Hole Oceanographic Institution (WHOI) Joint Program in Oceanography. But the phytoplankton Edwards and colleagues studied—diatoms—are different. When stressed, diatoms release bioactive molecules known as polyunsaturated aldehydes (PUAs). The researchers found that these molecules kick the bacteria’s metabolism and CO2 respiration rates into hyperdrive—like skinny weightlifters after a steroid shot. The bacteria start devouring the falling particles like they are at an all-you-can-eat buffet. They significantly reduce the amount of sinking detritus while releasing more CO2. Molecules like PUAs are termed “infochemicals,” and Van Mooy said this research is one of the first to show how infochemcials affect what happens to CO2 in the ocean. “The depth of organic carbon sinking is important, as nearly a quarter of CO2 from fossil fuels are in the deep ocean because of these mechanisms,” said Van Mooy. “For more than half a century, people have been trying to understand why carbon sinks more here and less there. These molecules [PUAs] are telling us they have a role to play in all of this.”
Tiny compounds, planetary impacts
Edwards, Van Mooy, and Bidle went to sea to collect and analyze particle samples from several locations across the North Atlantic, including the Sargasso Sea, the subarctic North Atlantic near Iceland, and the western North Atlantic near Massachusetts. The spatial coverage was important, Van Mooy said. “We know that there’s more sinking carbon in some places and less in others, so we wanted to understand the distribution better across different ocean regions,” Van Mooy said. To collect the particles, 6-foot-wide, funnel-shaped sediment traps were submerged 150 meters down (picture huge traffic cones dunked upside down in the ocean) for six to 24 hours, depending on how rough or calm the seas were. Once the traps were brought back to surface, the scientists incubated collected particles with PUAs and analyzed changes in bacteria’s respiration over a 24-hour period. “The results were very surprising,” Edwards said. “Very rarely do you see organisms respond positively to PUAs. In fact, in higher concentrations, they often have a toxic effect, causing a decrease in phytoplankton growth rates and mutations. But we saw an increase in CO2 production rates, enzyme activity, and bacterial cell growth.” The scientists also found much higher concentrations of PUAs within the sinking particles than had been previously been observed in the water column. “This suggests that sinking particles are ‘hotspots’ for PUA production,” Edwards said. “These scientists have uncovered yet another nuance that may affect the efficiency of the biological pump to remove carbon from surface waters,” added David Garrison, program director for National Science Foundation’s Biological Oceanography Program.
Arkansas traveler
Long before delving into the nitty-gritty of marine microbes, Edwards majored in biology, sharing lectures with “a lot of nervous pre-med students” at Hendrix College in the landlocked state of Arkansas where she grew up. “Arkansas is obviously not known for ocean sciences, but my parents were really into scuba diving when I was a kid, so that had a strong influence,” Edwards said. “When I was in high school, I got the chance to go out on a naval oceanography office ship and see what marine scientists actually do.” During college, she got her first lab job at the Mount Desert Island Biological Laboratory in Maine studying estuarine fish. “Bethanie had a neat background in both biochemistry and oceanography, which are the two types of expertise you need to do this kind of work,” Van Mooy said. She arrived at WHOI in 2010, shortly after the Deepwater Horizon disaster and was immediately put on a project examining how marine microbes consumed oil spilled in the Gulf of Mexico. “I thought conservation was really interesting,” Edwards said. “But ultimately, I wanted something that would have more of an impact. When I learned about the role oceans play in climate and the pressing issue of climate change, I just kind of went with that and fell into this chemical oceanography realm where, instead of studying things like E. coli, I’m now studying marine microbes.” Edwards sees the research as a steppingstone toward a clearer understanding of CO2 absorption in the ocean and the efficiency of the biological pump in the vast planetary cycle that circulates carbon through air, land, ocean, and living things. “By gaining more detailed knowledge about the intricate interactions of marine microbes,” she said, “we can paint a more complete picture of how the carbon cycle works, the positive and negative feedbacks, and the implications for global climate.” The research was funded by the Gordon and Betty Moore Foundation, with additional support from the National Science Foundation and the Office of Naval Research.
From the Series
Slideshow

Slideshow
 Bethanie Edwards, a graduate student in the MIT-WHOI Joint Program in Oceanography, and her Ph.D. advisor Ben Van Mooy, have found chemical compounds that have planet-scale ramifications. The compounds, called polyunsaturated aldehydes, or PUAs, are released by stressed phytoplankton. They stimulate bacteria to speed up their decomposition of the phytoplankton. As a result, more carbon dioxide is released back from the ocean to the air. That diminishes the ocean's ability to reduce the amount of atmospheric carbon dioxide, which traps heat and causes climate change. (Tom Kleindinst, Woods Hole Oceanographic Institution)
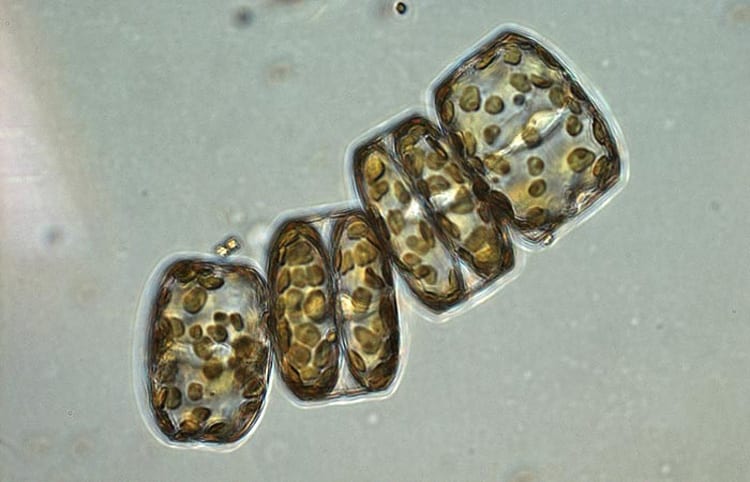
Bethanie Edwards, a graduate student in the MIT-WHOI Joint Program in Oceanography, and her Ph.D. advisor Ben Van Mooy, have found chemical compounds that have planet-scale ramifications. The compounds, called polyunsaturated aldehydes, or PUAs, are released by stressed phytoplankton. They stimulate bacteria to speed up their decomposition of the phytoplankton. As a result, more carbon dioxide is released back from the ocean to the air. That diminishes the ocean's ability to reduce the amount of atmospheric carbon dioxide, which traps heat and causes climate change. (Tom Kleindinst, Woods Hole Oceanographic Institution)- To collect the particles of sinking phytoplankton, the scientific team submerged 6-foot-wide, funnel-shaped sediment traps 150 meters deep at several locations across the North Atlantic Ocean. The scientists later incubated the collected particles wtih bioactive molecules known as polyunsaturated aldehydes. The PUAs stimulated marine bacteria to decompose the phytoplankton particles. (Suni Shah, Woods Hole Oceanographic Institution)

