To Free a Tangled Whale
... and other recent findings by WHOI researchers
To Free a Tangled Whale
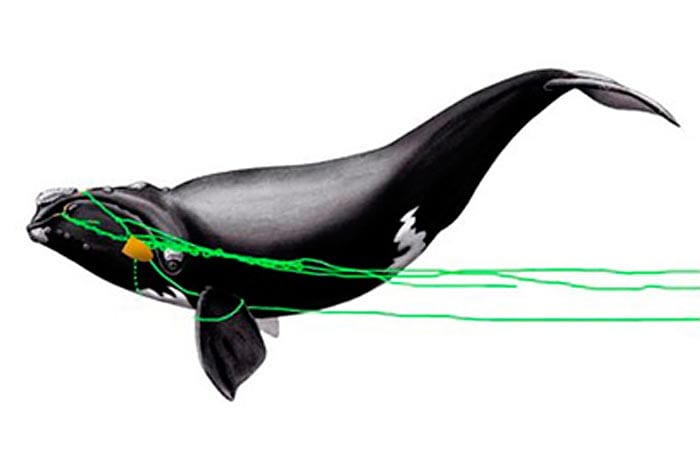
Scientists successfully used a new sedative delivery system for the first time on a large whale in the wild. It calmed the 40-foot, 40,000-pound whale so that rescuers could approach safely by boat and cut away fishing gear wrapped around its head.
Over the past 10 years, Michael Moore, a biologist at Woods Hole Oceanographic Institution, has collaborated with the NOAA National Marine Fisheries Service and veterinary schools at the University of Florida and the University of Wisconsin to develop a sedation system to make it easier—on both whales and rescue teams—to extricate whales entangled dangerously in fishing lines.
Entanglement in commercial fishing gear can cause slow, painful death, and it is a particular concern for endangered North Atlantic right whales. Fewer than 400 remain, and 70 percent of their population exhibits scars from fishing gear.
“The typical success rate for freeing right whales from fishing gear is about 50 percent, due largely to the difficulties in getting close enough to cut the entangling gear,” said Jamison Smith, NOAA’s East Coast project leader for whale disentanglement. “We hope this new technique can improve the overall safety of the operations, as well improve the chances of the whales’ survival.”
The new sedation delivery system, built by Trevor Austin of Paxarms New Zealand, comprises a 12-inch needle and a syringe that uses compressed air to inject drugs into whales’ muscles. The researchers had to calculate a dosage for large whales, based on experience sedating animals in captivity, starting at a low dose until they found a safe and effective level.
The entangled whale was first sighted off Georgia on Jan. 14, 2009, by an aerial survey team, which noted multiple lengths of heavy line cutting into the whale’s upper jaw and left lip and trailing behind the animal. The whale evaded all attempts by rescuers to cut the lines on Jan. 22. They tried again the next day, using a sedation dose, but the whale was not sedated enough to be more approachable. Another disentanglement attempt failed on Feb. 1.
On March 5, the disentanglement team tried again, increasing the dosage used on Jan. 23. The sedative appeared to cause the whale to take shallower, more frequent breaths, but the animal continued to evade the boat’s attempts to approach it. On March 6, the team increased the dose further. An hour after the sedatives were injected, the animal no longer evaded the boat and tolerated close approaches, allowing the team to remove 90 percent of the remaining rope. The whale has not been sighted since, Moore said, “so I don’t know the outcome of this case.”
Moore welcomed “this novel and exciting new tool in the large whale disentanglement toolbox.” But, he noted, “it does not address the underlying problem of how to enable fisheries to pursue a profitable business without jeopardizing the survival of endangered species such as the North Atlantic right whale.”
—Stephanie Murphy
IMAGE CAPTION: Top: A rescue team couldn’t get close enough to try to cut away fishing gear wrapped over the head of an endangered North Atlantic right whale
RELATED LINKS:
» Workshop report on whale disentanglement
» Workshop report: Reports from a disentanglement workshop at WHOI.
» Michael Moore’s Lab: Michael Moore is a marine mammal biologist and veterinarian.
Dust blown from land could be toxic in the sea
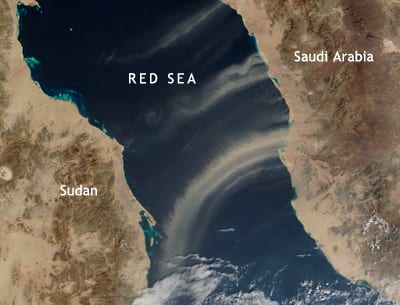
For the first time, scientists report that toxic particles blowing off continents can have deadly impacts on some marine phytoplankton. High concentrations of airborne copper deposited in the northern Red Sea could be responsible for the toxic effects that researchers found on phytoplankton there, according to a study published March 24, 2009, in Proceedings of the National Academy of Sciences.
The researchers went on to analyze data about various sources of copper in aerosols (airborne particles), global distributions of aerosols, and aerosol deposition rates. (Most of the copper deposits in the ocean come from natural sources of dust; manmade sources accounted for roughly 40 percent.) They developed models that suggest certain areas—such as the Bay of Bengal and downwind of South and East Asia—are particularly at risk for the effects of copper deposition on ocean ecosystems.
Other pollutants in aerosols could also have toxic impacts on marine life, the scientists said. The research team, led by Adina Paytan from the University of California, Santa Cruz, included scientists from Woods Hole Oceanographic Institution (WHOI), Cornell University, the U.S Geological Survey, and Interuniversity Institute of Marine Sciences in Israel.
“We need to do more extensive sampling of aerosols over larger areas and over greater lengths of time,” said WHOI geochemist Scott Doney. “We also need to start thinking about varying responses from different species in the planktonic community.”
This research was supported by grants from NASA, NATO, and the National Science Foundation.
IMAGE CAPTION: Scientists have found that toxic particles in fine sediments blown off continents can affect marine life. Above, a dust plume blows off Saudi Arabia over the Red Sea. The image was captured by the Moderate Resolution Imaging Spectroradiometer (MODIS) on NASA’s Terra satellite on Jan. 15, 2009 (Courtesy of MODIS Rapid Response, NASA Goddard Space Flight Center).
RELATED LINKS:
» Dust deposited in oceans may carry elements toxic to marine algae: UC Santa Cruz
Are seafloor vents a source of iron for organisms?
Fluids spewing from seafloor hydrothermal vents contain about a million times more iron than seawater. When the fluids hit oxygen-rich seawater, scientists thought the iron immediately oxidizes into a form that has as much nutritional value for organisms as chewing a rusty nail has for patients with anemia.
In a paper published in the March 2009 issue of Nature Geoscience, Brandy Toner and colleagues report the unexpected discovery that some iron spit out of hydrothermal vents remains in a form that organisms can use. The iron appears to stick to organic matter, and the chemical interaction with carbon seems to protect the iron from oxidizing, said Toner, a NASA postdoctoral fellow at WHOI when the research began and now an assistant professor at the University of Minnesota.
Toner and colleagues used the Lawrence Berkeley National Laboratory’s Advanced Light Source synchrotron to focus X-beams on particles in sediments collected at a Pacific Ocean vent—creating elemental maps of the particles on micrometer and nanometer scales. The technique unexpectedly revealed a form of iron called iron(II), a delectable treat for iron-starved organisms in the ocean. Exactly how the iron(II)-laden carbon particles might interact with the marine food web is still to be determined.
“So the question becomes, what are those [carbon-containing) organic compounds?” asked co-author Chris German, a geochemist at WHOI. “Are they organic compounds like in oils and tars, or is it actually the stuff of life? This paper opens up a whole new line of research and asks a new set of questions that people didn’t know they should be worrying about until now. A bit of work on a tiny nanometer scale can force you to ask questions of global significance.”
Research support also came from the National Science Foundation and the WHOI Deep Ocean Exploration Institute.
IMAGE CAPTION: Seafloor hydrothermal vents spew hot fluids filled with minerals, including iron. To their surprise, scieintists discovered that some of the iron does not get oxidized when it hits oxygen-rich seawater, but instead remains in a form that organisms can use. (Alvin Group, Woods Hole Oceanographic Institution)
RELATED LINKS: