Rites of Passage for Juvenile Marine Life
Learning from the life-or-death journeys of barnacle, lobster, and clam larvae
The childhood of a barnacle is fraught with challenges. It hatches in shallow waters close to shore as a tiny larva, no bigger than a speck of dust. Currents sweep it to deeper, choppy waters, sometimes miles offshore. In these proving grounds each larva floats, at the mercy of hungry fish and swift ocean currents.
Billions of larvae—including fish, lobsters, clams, starfish, and sea cucumbers—begin life this way. Only a few survive and return to shore, where they settle on rocks or sandy seafloor to become adults.
Why larvae make their offshore journey remains unclear, but we are beginning to uncover the intricacies of their return trip—learning how waves, currents, eddies, tides, and other phenomena bring larvae back toward the shore. With more insight into the ocean’s role in this essential phase in the life cycle of these species, natural resource managers can devise better strategies to sustain healthy, vibrant habitats for marine life.
For example, ocean circulation may explain why populations of lobsters or clams are sparse in some rocky coastal habitats that appear ideal. Prevailing ocean currents may never deliver larvae to their promised land. Similarly, it would be futile for coastal managers to create marine reserves or shellfish beds in areas where larvae are doomed by currents to be carried so far offshore that they cannot return.
Telltale clues in murky waters
My interest in larval research was sparked in 1988 during a scuba diving class in La Jolla, Calif., where I had just begun my doctoral studies at Scripps Institution of Oceanography. During a dive close to shore, I spotted a layer of brown water about 10 meters (33 feet) down. When I swam into it, the water felt very cold.
The next day I returned and swept a net through this brown water. When I analyzed my catch in the lab, I found the color came from patches of maturing larvae ready to settle near the shore. These observations hinted at a mechanism for transporting larvae toward the beach.
Near the coast, warm fresh water often collides with colder, saltier water. These different bodies of water have different densities, so they tend to remain relatively distinct. In many ways, the boundary between these water masses behaves like the boundary between two other “fluids”—the sea surface and the atmosphere. Just as waves form and propagate on the ocean surface, waves also form within the ocean, along the boundaries between warm, light water and cold, dense water.
My observations in scuba diving class suggested that larvae were being transported toward shore in cold-water waves moving below the sea surface. But these waves, called internal bores, told only half of the story.
Coastal fronts
I spotted evidence for the other half of the puzzle on my way home from a seaside party. Looking at the Pacific Ocean, I saw lines of foam, seaweed, and plastic debris forming parallel to the shore and stretching several hundred feet across the sea surface. Most beachgoers have probably seen these features, which form at the intersection where warm and cold water masses meet. Scientists call them coastal fronts.
The full picture of the larval transport process fell into place. As ebbing tides move water over seafloor obstacles, they create internal bores, which transport large volumes of cold water from deep offshore areas toward the shore. The incoming wave of cold water pushes warmer nearshore waters out to sea. A few hours later, the heavier cold water sinks and recedes offshore. Warmer, lighter water near the surface surges inshore and over the receding cold water, led by a vanguard of coastal fronts. Along with kelp and debris, the coastal fronts carry larvae ashore.
A rare wave within the ocean
In the years since those observations, I have studied various phenomena involved in larval transport along the coasts of southern California, northwestern Mexico, and Massachusetts. With funding from the National Science Foundation and the Office of Naval Research, I have investigated why some types of larvae accumulate in fronts, while others do not. I also have examined whether periodic ocean circulation changes such as El Niño have an impact on larval transport.
Many puzzles remain. For example, we know little about how currents in the nearshore environment connect with larger oceanic currents. If larvae are swept far from the shore of their birth, do they grow up somewhere else or are they lost altogether?
And while our work on internal bores has focused on larvae, other researchers have been interested in whether this process transports offshore deposits of sewage and nutrients back onto the beach.
Recent research has shown that internal waves come in various flavors with varying impacts on larvae. (Internal bores are but one flavor.) I am currently collaborating with Alberto Scotti, who studies fluid dynamics at the University of North Carolina, to investigate a rare type of internal wave in the ocean called a “wave of elevation.” As the name suggests, these waves have taller crests than others around them. Within each wave of elevation a strong circular flow of water moves horizontally, spinning any trapped particles like clothes in a washing machine.
These waves are rarely observed in nature, but we identified some in Massachusetts Bay. We suspect they may influence how larvae move to their adult settlements. As the wave moves toward land, larvae may become caught up in this rotation and concentrate together.
To test this, we are planning to conduct an experiment where we will deploy plastic-covered electronic drifters (about the size of soda cans) in areas with waves of elevation. We will record information about buoyancy and particle accumulation in the hope of finding out what larvae must do to catch a ride on these waves.
Surfing to turf
I have also conducted experiments in the controlled environment of the laboratory to mimic what I saw in the sea. With funding from the WHOI Rinehart Coastal Research Center, I collaborated with WHOI physical oceanographer Karl Helfrich, who studies the mechanics and fluid dynamics of the coastal ocean.
We used a rectangular tank bisected with a Plexiglas dam, filling one side with lighter fresh water, dyed blue, and the other side with heavier, saltier water. We added buoyant, peppercorn-sized nylon beads (representing larvae) to the fresh water. When we lifted the dam, a tongue of blue-dyed fresh water surged across the surface of the salty water. We watched as the beads accumulated behind this freshwater tongue.
Applied to the coastal zone, this experiment suggests that some species of larvae may accumulate behind—not in front of—coastal fronts and internal bores. These larvae surf the waves toward shore. However, we also found that the larvae of other species may be pushed ahead of an incoming front, like the dirt piled up in front of a bulldozer.
Swimming against the current
Whether larvae are pushed or pulled onshore turns out to make a difference. The collision of water masses of different densities creates powerful downward-flowing currents that threaten to sweep larvae into the depths. The larvae must resist this downward pull and continue moving forward.
They do this by swimming. Several weeks into their growth cycle, larvae develop appendages to swim. Some organisms, such as sea anemones, develop cilia—wiggly, hair-like protrusions that work like oars. Other species such as barnacles grow a single strong leg that functions like a flipper.
With Karl Helfrich and Claudio DiBacco, a larval ecologist at the University of British Columbia, I am now using a specially designed experimental chamber to determine how larvae swim when downward currents flow against them. We are interested because those larvae that do not swim fast enough will be left behind by the traveling front and will not reach the shore. Karl and I also have found that in order to resist the downward drag, larvae swimming ahead of a front have to swim faster than those swimming behind.
The challenges confronted by larvae do not end near land. We are also interested in what happens to them once they arrive close to shore. Frequently my colleagues and I leave our microscopes and laboratories and head to beaches, coastal lagoons, and rocky coastlines to study how larvae accumulate and mature. Some areas host thick carpets of juvenile organisms. Others have none at all.
In 2003, I visited a new research facility along the Pacific coast of Panama known as the Liquid Jungle Lab. While exploring a nearby estuary, I examined a clump of mangrove roots. The rocky, remote, and relatively pristine sanctuary is a habitat rich with food, so I suspected it would be equally rich with marine species. Yet I found surprisingly few organisms.
Very few people live near the lab, so it’s unlikely that pollution prevented colonization of the mangrove roots. I suspect that as rainforest leaves fall and rot, they leach naturally occurring chemicals into the coastal zone and kill any larvae. I plan to return and test this hypothesis because, despite their small size, larvae teach me a lot about life in our oceans.
—WHOI Science Writer Amy E. Nevala contributed to this article.
Slideshow

Slideshow
- A CLOSE LOOK AT BARNACLES—Tracy Pugh, a former research assistant in the WHOI Biology Department, records the growth of barnacles in Buzzards Bay on Cape Cod, Mass. Understanding how juvenile barnacles and other marine invertebrates are transported by waves, currents, eddies, tides, and other phenomena helps researchers devise strategies to sustain vibrant habitats. (Jesús Pineda, WHOI.)
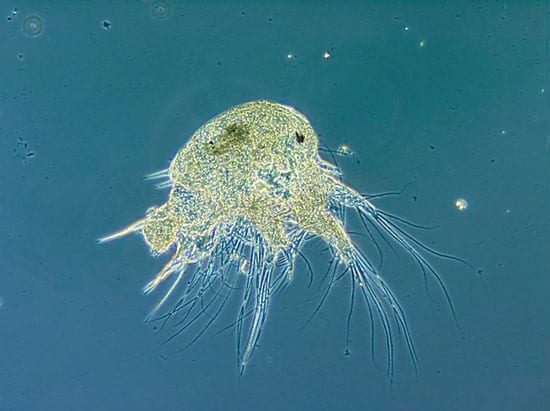
- LIFE WITH LARVAE—Hair-like appendages on this two-week-old barnacle larva churn the water to aid in feeding. (Fabian Tapia, WHOI. )
- After four to seven weeks, juvenile larvae settle along the shore and develop a hard white shell. (Todd Stueckle, WHOI.)
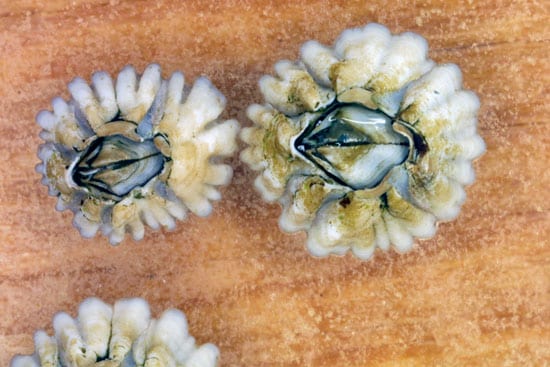
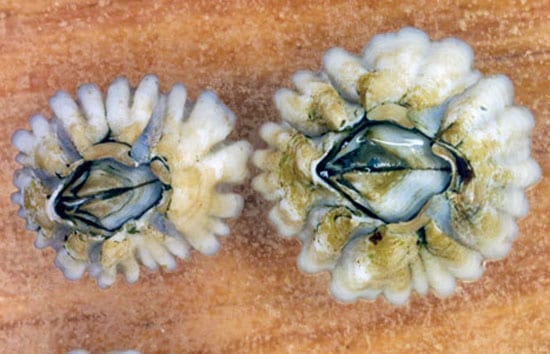
- Adult barnacles, about one year old, form plates to hold their body together and for protection. (Photo by Vicke Starczak, Woods Hole Oceanographic Institution)
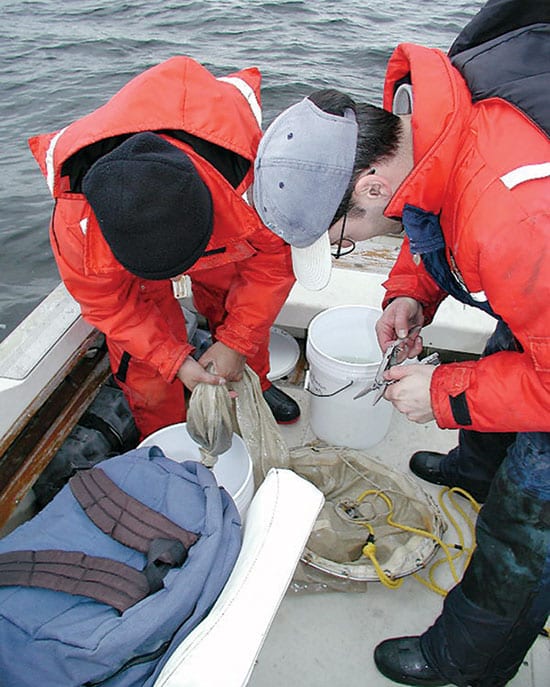
- MIT/WHOI Joint Program student Fabián Tapia (left) and WHOI Postdoctoral Scholar Claudio DiBacco use fine nets to sample patches of cyprid barnacle larvae (photo below) floating in Narragansett Bay, R.I. Larvae ride internal bores—wave fronts beneath the surface of the ocean—toward the shore when they are mature enough to settle on rocks and coastal structures. (Jesús Pineda, WHOI.)
- (Jesús Pineda, WHOI.)