A Faster Way to Better Reactions
'One-pot' approach could speed syntheses of new products
When we tell people that we are chemists, they occasionally crinkle their noses in a subtle but unmistakable expression of pain. Often this is followed by a retelling of the time they almost caused a fire or explosion when attempting to make soap, aspirin, or other products in chem lab.
Many factors go into chemical reactions, opening up many avenues for things to go wrong. On the other hand, myriad possible chemical reactions exist to synthesize new commercial and industrial compounds, from shampoo to silicon chips. Many potential reactions have still not been discovered.
It is big business and a major source of innovation to find new chemical reactions (or refine old ones) that combine different compounds with various catalysts at different temperatures to produce different amounts and assortments of products at different rates. Synthesizing chemicals is essential for a wide range of multibillion-dollar industries, from cosmetics to energy technology and pharmaceuticals. More than half of all new drugs developed over the last 30 years were synthesized in the laboratory. There’s a lot of money to be made or saved by inventing new chemical reactions or improving old ones to make exactly what you want in faster, better, cheaper, and greener ways.
In this quest, synthetic chemists study the mechanics underlying chemical reactions. They “road-test” countless variations in reactions to see what works and what works better—preparing, running, and analyzing thousands of reactions, one reaction at a time in one flask.
We have found a new way to analyze many reactions at one time in one reaction flask, or “pot”—considerably reducing the cost and effort to find innovative ways to manufacture useful chemical compounds.
‘Road-testing’ chemical reactions
If you were trying to devise a new recipe, you’d test different ingredients in different combinations, preparations, cooking temperatures, and other conditions. To test reactions, chemists run them through a gauntlet of what are called “scoping molecules.” Each scoping molecule has a specific known chemical structure with a slight chemical idiosyncrasy that distinguishes one from another—their sizes, for example, or something more subtle such as the distribution of electrons within the molecules. Using scoping molecules is a standard procedure that helps chemists identify precise molecules and chemical pathways that will hone the performance of their reactions.
The situation becomes more complicated when you add catalysts to the reactions, as is often the case. Catalysts are substances added to make reactions go faster. They often provide an alternative route for the reaction to proceed, effectively lowering the energy it takes to get a reaction going.
A classic example is the Nobel-prize winning Haber-Bosch process for combining nitrogen gas (N2) and hydrogen gas (H2) to make ammonia (NH3). Until a catalyst was found to make things happen, the reaction was so slow that it virtually didn’t occur. But this reaction changed the world, allowing vast and easy production of ammonia used in explosives such as TNT and in fertilizers. Identifying the subtle impacts of various catalysts to deter or enhance reactions or create very specific products also requires extensive testing. To this day, chemists are still seeking new and improved catalysts for this reaction.
To make matters even more challenging, many products from the reactions can be nearly the same compound, having the same chemical formula but differing only in their three-dimensional shapes. These are called chiral molecules, and they are non-superimposable mirror images of one another, like our left and right hands. This subtle difference in “handedness” can create compounds with very different properties. One example is the molecule carvone: One mirror image smells like spearmint and the other like caraway seeds.
Think of children’s Tinker Tot toys. The single difference is the direction in which the hydrogen (H) points: inward on the spearmint (indicated by dotted line), outward on the caraway (indicated by solid line).
Another example occurred in the 1950s and 1960s with thalidomide, a compound that effectively treated morning sickness in pregnant women. Tragically, it was recognized too late that the other mirror-image form of thalidomide was a teratogen that led to severe birth defects.
So once again synthetic chemists have to test their reactions rigorously with scoping molecules to determine not only what compound is produced, but how much is left-handed versus right-handed.
Historically, getting accurate measurements of handedness and other road-test results requires many tests with many scoping molecules. The more molecules tested, the more precise the results. A recent paper on a synthesizing reaction in a high-profile journal included results for 80 different molecules. That’s 80 different reactions separately executed and analyzed just for this one study!
With new catalysts—many proprietary—being made every day, chemists perform thousands of reactions every year. Analyzing each one separately creates a major bottleneck.
Analyzing the mechanics of reactions
One traditional method used is called gas chromatography. The instrument consists of a syringe injector, a column, and a detector. The column consists of a glass tube almost 200 feet long and as thin as the lead in a mechanical pencil. The tube’s interior has a chemical coating. A sample composed of a mixture of compounds is injected into one end of the column.
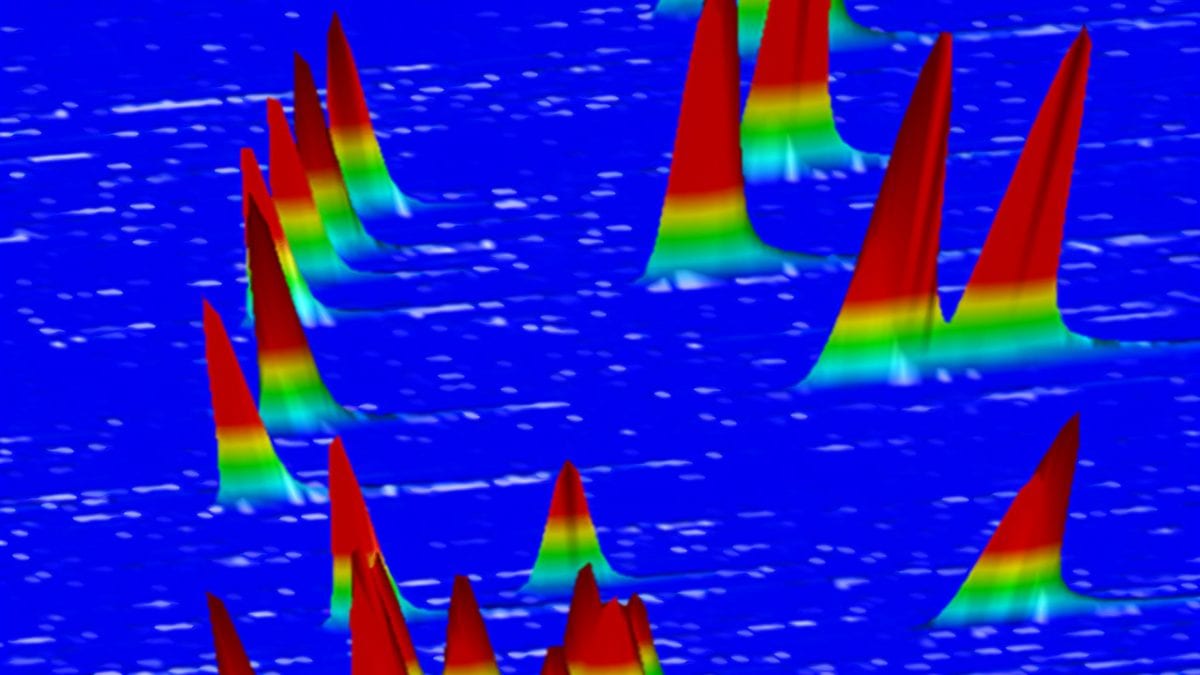
Each compound interacts differently with the chemical coating and proceeds through the coating at its own pace. Depending on its unique properties, each compound emerges separately out the other end sooner or later, and is detected. As each compound emerges, it appears as a peak on a gas chromatogram that looks like this:
For our experiment, we put nine scoping molecules into one pot in the presence of a catalyst.
Each molecule produced two products, one left-handed and one right-handed (18 compounds). And some of each molecule also didn’t react and was left over (9 compounds). So at the end of the reaction, a total of 27 compounds were in the pot.
Here’s another look at the traditional gas chromatograph for these reactions (below). You’ll notice there are only 18 peaks. Even some of those are hard to distinguish—look at the third hump from the left, which actually has three tiny mini-peaks, representing three compounds, on top of it.
No matter what, there are definitely not 27 peaks. Why? Because some of these compounds are so similar, the difference in the times they emerge from the tube is tiny. They emerge nearly simultaneously (or “co-elute,” as we chemists call it). The peaks representing them overlap and you can’t distinguish some products from one another. As a result, some data is obscured and chemists can’t get a complete and precise picture of what happened.
We believe our new method, based on research done on oil spills, can reduce the work needed to do these reaction analyses.
Multi-dimensional gas chromatography
Oil can be composed of thousands of individual chemical compounds. To distinguish them, we have used a technique called comprehensive two-dimensional gas chromatography (GCxGC). Beyond the initial separation described above, we further distinguish the compounds by sending them through a second column that separates them on the basis of a second characteristic or dimension: their polarities, or hydrophobicity (how much they are repelled versus attracted to water).
With our colleagues Alicia Wright at Western Washington University and Robert Nelson at Woods Hole Oceanographic Institution, we conducted an experiment in which we added an extra level of separation. We also ran the different compounds through a kind of tube that separates them on the basis of their three-dimensional shapes, or chirality. Think of this tube as a staircase filled with lots of different-shaped stuff in different places on the stairs. Now think of each compound as a different-shaped sofa. It’s going to take longer or shorter for the movers to negotiate certain-shaped sofas up the stairs versus other-shaped sofas. So different chiral compounds will emerge, be detected separately, and form distinct peaks on gas chromatograms.
When all nine scoping reactions were performed in one pot and analyzed by GCxGC, the gas chromatogram looked like this one below. For reference, the traditional gas chromatogram, showing only 18 peaks clearly, is to the right in white. But the multi-dimensional chromatograph successfully displays 27 distinct peaks.
We made the “scoping” of a reaction nine times easier: Instead of nine different flasks, nine different “work-ups” to prepare for analysis, nine sets of waste for disposal, and nine different injections on a traditional gas chromatograph that can make a chiral separation, we did it with one.
In some ways, this approach is like making a stew with meat, potatoes, carrots, and onions. We worried that our “chemical multitasking” might be registering not carrots, but carrot-flavored potatoes, or onion-flavored meat instead of onions. Just to be sure, we checked the results for our one-pot reaction with results from nine separate reactions, and we are assured that the one-pot method has the power to distinguish products precisely.
We published our study in the May 2016 issue of the Journal of Organic Chemistry published by the American Chemical Society. We think that the method can be used by a wide spectrum of synthetic chemists to make their work faster, greener, and cheaper. Investigating these reactions in this manner may also reveal insights not as evident with the one-molecule-at-a-time approach. Sometimes it’s not what you do, but how you do it, that spurs innovation.
Related Articles
- Our eyes on the seafloor
- 4 Potential Solutions for Corals in Crisis
- The hypoxic reef
- WHOI scientists discuss the chemistry behind Sri Lanka’s flaming plastic spill
- An ocean of opportunity
- The ocean has heartburn. Is relief on the way?
- Science RoCS Initiative responds to need for increased ocean monitoring
- A new ocean soundscape
- Putting the ‘nuclear coffin’ in perspective
See Also
- A One-Pot/Single-Analysis Approach to Substrate Scope Investigations Using Comprehensive Two-Dimensional Gas Chromatography (GCxGC) Study in The Journal of Organic Chemistry by Gregory W. O’Neil, Robert K. Nelson, Alicia M. Wright, and Christopher M. Reddy
- Greening the Chemical Industry Western Washington University news release
- Greg O'Neil
- Chris Reddy
- Jet Fuel From Algae? Oceanus magazine
