A Journey to the Ocean’s Twilight Zone
A conversation with marine biogeochemist Ken Buesseler
You are about to enter another dimension. You’re moving into a place of both shadow and substance, of things and ideas; a journey into a wondrous part of the ocean, whose boundaries are 300 to 1,600 feet (100 to 500 meters) below the surface, where sunlight fades into blackness. There’s no signpost up ahead, but your next stop is the ocean’s twilight zone.
No, your host won’t be Rod Serling; he’s unavailable, and besides, he was no expert on how the oceans work. For this twilight zone, your best guide is Ken Buesseler, chair of the Marine Chemistry and Geochemistry Department at Woods Hole Oceanographic Institution. As chief scientist of a research project called VERTIGO, he mustered an arsenal of instruments and a small army of ocean scientists from many institutions and disciplines— biologists, chemists, physical oceanographers, and engineers—and led research cruises in 2004 and 2005 to explore this dim and dimly understood region of our planet.
What’s lurking in the ocean’s twilight zone?
We now know that many organisms call the twilight zone their home. But how do they make a living in this zone where no plants grow? What do they eat? What adaptations do deep-living zooplankton and fish have that enable them to survive, reproduce, and flourish in the twilight zone?
There’s a thin skin on the top of the ocean, where light penetrates, photosynthesis happens, and phytoplankton grow. Some zooplankton and fish in the twilight zone migrate daily to surface waters to feed on phytoplankton, or they eat each other. But many critters in the deep ocean or on the bottom eat detritus—dead phytoplankton or the feces of zooplankton, for example—that sinks from the surface like manna from heaven. It’s called “marine snow.” It’s the food supply for the deep sea.
Fascinating for biologists, but what’s in it for a chemist?
We think of the surface waters as a factory that produces sinking particles, and these particles are the primary means by which materials—carbon, for example—get from the surface to the deep ocean. There are really only two ways for materials to get there—either by currents that sink to the deep ocean (but that is a slow process and occurs only in a few regions, near the Arctic and Antarctic), or by hitching a ride on sinking particles.
Personally, I’ve never been that interested in theoretical chemistry per se—reaction rates, kinetics, thermodynamics—but in measuring and tracking how chemicals move through the oceans, because that can tell you how the Earth, the ocean, and atmosphere work.
My specialty is measuring radioactive isotopes in the ocean, especially thorium-234, which is “sticky”—it tends to bind to sinking particles and marine snow. It also acts as a clock because every 24.1 days, half of it decays. So by tracking thorium-234, we can measure how fast elements—particularly carbon—move through the ocean and how they are transformed by phytoplankton and zooplankton along the way.
Is that how your lab got the nickname “Café Thorium”?
Once we discovered the usefulness of thorium-234, the problem was that with existing methods, we could only get maybe 10 to 20 measurements on a research cruise. I figured out better ways to collect and process samples for thorium analyses and get hundreds of measurements quickly.
That jumped things into high gear, and we began collecting samples on many cruises all over the world. We streamlined the process so that one person could physically do the job—if he or she could stay awake enough hours.
Caffeine was helpful to make that happen. So along with our scientific gear, we packed our own espresso makers and good coffee, so that at 3 a.m. when we were still working, we did not have to drink hideous coffee that had been on the stove since dinnertime. We designed a logo for the lab with a thorium atom, a coffee cup, and a bolt of lightning. We had a little fun and made T-shirts with our logo. And we kept that tradition going for…well, we still do.
Why have you focused on the twilight zone?
It’s a critical link between the surface and the deep ocean. We’re interested in what happens there, what sinks into it and what actually sinks out of it. It’s an important component of the oceanic food web, and we’re also interested in how much carbon is exported into the deep ocean. The oceans are taking up about half the carbon dioxide released due to fossil fuel burning. Carbon dioxide is a greenhouse gas, so the more that is in the atmosphere, the warmer our climate becomes. Unless the carbon that gets into the ocean goes all the way down into the deep ocean and is stored there, the oceans will have little impact on the atmosphere and the climate.
How does that work?
Most of the surface ocean is like your lawn. When it grows, photosynthesis transforms carbon dioxide from the atmosphere into organic carbon in the grass. When you mow your lawn or winter comes along, that organic carbon decomposes back into inorganic carbon and carbon dioxide. It recycles itself efficiently and produces no net changes in carbon dioxide levels in the atmosphere.
Most of the marine plants, or phytoplankton, that grow and die in the surface ocean just decay away, and carbon is exchanged back and forth with the atmosphere. But any carbon in these plants that sinks through the twilight zone will end up in a large reservoir of deep-ocean waters that don’t come back to the surface and can store carbon dioxide for hundreds of years. That has an impact on climate. And it all goes back to how particles are cycled and transported through the twilight zone.
That explains VERTIGO, which—in the oceanographic tradition of constructing tortured acronyms—stands for VERtical Transport In the Global Ocean. Tell us about it.
The key questions we are studying are: Where do marine particles come from? How do marine plants and animals create and destroy particles in the ocean? How quickly do particles sink? How deep do they go? Are all marine particles the same? Will climate changes change the forecast for marine “snow”?
As on land, the ocean has different ecosystems with very different conditions and fauna. So we mounted research cruises to places with different characteristics: off Hawaii in 2004 and in the northwest Pacific near Japan in 2005.
The scientists onboard included chemists, biologists, and physical oceanographers, because the phenomena we’re studying require all of those to understand it. And we brought out all sorts of equipment, including specially designed instruments, to make measurements of what’s happening in the twilight zone. On the last cruise, we had something going in or out of the water almost every hour, 24 hours a day, over 20 days.
What kinds of instruments?
We had instruments called CTD rosettes that collect water samples and use sensors to measure water temperature, salinity, oxygen and the number and types of marine particles. We deployed our unique new vehicles, the Neutrally Buoyant Sediment Traps, which work like no other trap to accurately capture sinking material in the ocean. (See “Swimming in the Rain.”) We used other traps from drifting surface buoys, including one that separates particles by sinking speed and one that collects microscopic-scale photos of sinking marine particles. We collected plankton samples with specialized net systems and chemical samples with large-volume filtration systems.
We’ve collected an amazing amount of new and unique data and samples. So, more than from any prior study, we should get a three-dimensional view of changes over time in the twilight zone.
Any preliminary findings?
We found a big contrast in the two locations we studied. Off Hawaii, we found that 80 percent of the particle carbon was recycled back into dissolved carbon by the time it sank to 500 meters (1,600 feet), and so most of the carbon didn’t make it down to the deep ocean. In the northwest Pacific, 50 percent actually made it down, which is much higher than previous estimates.
We really don’t know yet why there is this big difference. It may have to do with lower temperatures in the northern Pacific, which slow down the bacterial breakdown of organic carbon. It may also have to do with the northwest Pacific having lots of silica, which plankton incorporate into their shells. Those shells are heavier than those made by critters off Hawaii, so they may sink faster. Off Hawaii, particles may sink more slowly, giving bacteria more time to break them down. This was something that we scientists kind of knew in our hearts, but we hadn’t been able to measure it well before.
One of the amazing things about ocean sciences is that we are still in a “discovery” stage. Every time we drop our water bottles in and look at the data, there are still surprises.
Slideshow

Slideshow
 To stay awake analyzing as many thorium isotope samples as possible, Ken Buesseler and colleagues brought espresso makers and good coffee out to sea, earning his laboratory the nickname ?Cafe Thorium.? (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
To stay awake analyzing as many thorium isotope samples as possible, Ken Buesseler and colleagues brought espresso makers and good coffee out to sea, earning his laboratory the nickname ?Cafe Thorium.? (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)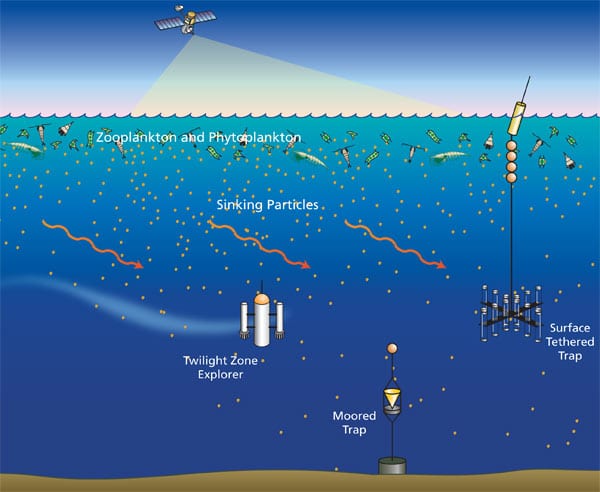 Particles sinking from sunlit surface waters through the ocean?s dimly lit twilight zone are swept sideways by currents. Conventional moored or tethered traps designed to catch the particles are like ?rain gauges in hurricanes,? said WHOI biogeochemist Ken Buesseler. He and engineer Jim Valdes are designing a new-generation neutrally buoyant untethered vehicle called the Twilight Zone Explorer, which will be swept along with the currents. It will surface periodically to relay data via satellite. (Illustration by Jack Cook, Woods Hole Oceanographic Institution)
Particles sinking from sunlit surface waters through the ocean?s dimly lit twilight zone are swept sideways by currents. Conventional moored or tethered traps designed to catch the particles are like ?rain gauges in hurricanes,? said WHOI biogeochemist Ken Buesseler. He and engineer Jim Valdes are designing a new-generation neutrally buoyant untethered vehicle called the Twilight Zone Explorer, which will be swept along with the currents. It will surface periodically to relay data via satellite. (Illustration by Jack Cook, Woods Hole Oceanographic Institution) This "quilt" of microscopic ocean organisms collected during the 2005 VERTIGO cruise to the North Pacific was created by Mary Wilcox Silver, professor of oceanography at the University of California, Santa Cruz. (Mary Wilcox Silver, University of California, Santa Cruz)
This "quilt" of microscopic ocean organisms collected during the 2005 VERTIGO cruise to the North Pacific was created by Mary Wilcox Silver, professor of oceanography at the University of California, Santa Cruz. (Mary Wilcox Silver, University of California, Santa Cruz)
Related Articles
- An immersive twilight zone exhibit
- Can the twilight zone be fished responsibly?
- Five big discoveries from WHOI’s Ocean Twilight Zone Project
- Experts gather to discuss the ocean’s super-powered carbon pump
- A new way to track marine snow ‘blizzards’
- AI in the Ocean Twilight Zone
- Edie Widder: A light in the darkness
- How to speak “Ocean”
- In the Ocean Twilight Zone, Life Remains a Mystery
Featured Researchers
See Also
- Cafe Thorium
- VERTIGO Cruises
- Swimming in the Rain from Oceanus magazine
- Sediment Traps from the WHOI Ocean Instruments Web site
