
Testing the Waters and Closing Beaches
Researchers seek faster, better ways to detect harmful bacteria
On a warm, tranquil evening this summer, Falmouth resident Annette Hynes took a friend down to Wood Neck Beach. It is one of Annette’s favorite local beaches, with a long, lovely shallow slope, a grassy marsh and dunes, and rocks covered in periwinkles. That evening the women slipped into the cool water, finding it totally relaxing.
Until the next morning.
No, there was no rising crescendo of ominous chords and no great white shark. But something was there: silent, jawless, and very, very small.
Annette heard it from her roommate, who found out from their neighbor: “Did you hear they closed the beach?” Analyses of water samples—taken a day earlier at Wood Neck Beach—showed excessive levels of bacteria, indicating that the waters were polluted with sewage or fecal matter.
Bacteria that are completely harmless to humans are naturally abundant in ocean water. Beaches are closed when bacteria that shouldn’t be there show up. That has been happening more frequently, as harmful bacteria flow in with stormwater, or fall into the water with bird or animal droppings. When this occurs, swimmers could be splashing around with microbes capable of causing upset stomachs, diarrhea, rashes or earaches.
Beach closures are rising
To determine whether water quality meets standards for safe swimming, health officials currently sample water at beaches once a week to measure levels of “indicator” bacteria. In marine waters the indicators are the enterococci, a group of bacteria that commonly reside in the guts of animals, including humans. These intestinal bacteria make their way into the outer world via feces that are teeming with life—bacteria actually comprise one-third the weight of fecal matter. High levels of enterococci at the beach indicate the waters may also contain other disease-causing microbes that are present in sewage but are more difficult to detect.
Finding these bacteria and figuring out whether human health may be at risk is an increasingly important task. Last year there were 22,571 days of beach closures or advisories nationwide—second only to 2006 for the highest number of closures in the last 18 years, the period in which the National Resource Defense Council (NRDC) has kept track of the data. Indicator bacteria surpassed health standards 71 percent of those days. Stormwater carrying pollution to swimming waters was responsible for precautionary closures on 25 percent of those days.
In Massachusetts, 3 percent of water samples exceeded the bacterial standard in 2007, down from 4 percent in 2005 and 2006, according to the NRDC. On Cape Cod, more than 95 percent of the beaches closed because of high bacteria levels met health standards and were reopened on the following day, according to local public health departments.
But the task of measuring indicators and making closure decisions is fraught with complications and limitations, ranging from the methodology of detection to the unique survival strategies of the bacteria.
Counting bacterial colonies
To protect human health, the U.S. Environmental Protection Agency has mandated that officials monitor the prevalence of enterococci in the water during the bathing season. How do they monitor something so small?
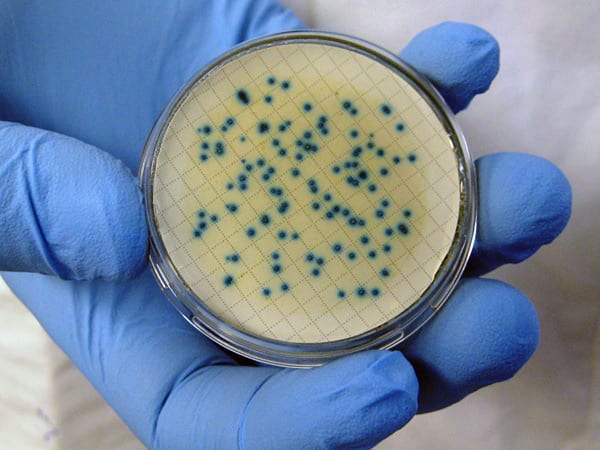
Filters separate the bacteria from a precise amount of water. Then the bacteria are placed on a gel infused with nutrients and chemicals designed to promote the growth of enterococci and to suppress the growth of other microorganisms. Left to their own devices in an incubator, the single cells isolated on the filter grow explosively, forming colonies visible to the naked eye.
The following day the colonies are counted, and if they exceed the safety standard set by the EPA (104 “colony forming units,” or CFUs per 100 milliliters of seawater), the beach is closed to swimmers. Often if a beach has high levels of indicator bacteria, it will immediately be tested again to see if swimmers can be allowed back in the water. That was the case at Wood Neck: After measuring 114 colony forming units and posting a closure decision, water was specially collected the next day and yielded only 20 colony-forming units, well within health standards.
However, the long incubation time required to grow the colonies creates an unavoidable lag in decision-making. People may have been swimming for two days before the colonies are counted and before officials make a decision to close or open the beach. And since contamination is often transient, by the time the beach is closed, the waters already may be perfectly safe for swimming.
A complicated picture
The way water is sampled is also critical to interpreting results. With current practices, samples may not be collected frequently enough under fast-changing circumstances at beaches to detect the bacteria harmful to beachgoers. Between pounding waves, swirling waters, harsh sunlight, and ebbing and flooding tides, it is difficult to know whether a bucket of water is representative of the bathing water over the course of a single day, not to mention over the course of a week. Management decisions are often based on a single water sample taken at one point in time. However, studies led by Alexandria Boehm, a researcher at Stanford University, suggest that amounts of indicator bacteria can vary extensively in water samples collected at the same spot, even on timescales of days or hours.
Another part of the picture is that once these bacteria get into the coastal ocean, they are tenacious and try to survive any way they can in their new environment. Only a few lucky bacteria might make it into a swimmer’s body, multiplying and making him or her sick, but there are other ways to stick around at the beach. Bacteria cling to available surfaces such as seagrasses, algae washed ashore, or even to the bodies of miniscule plankton, animals too small to see with the naked eye.
There’s also evidence that indicator bacteria can survive in beach sand and can later be resuspended into the water. That means that if you are sampling indicator organisms, there’s a chance they aren’t actually from recent pollution. It’s unclear if disease-causing microbes stick around the same way indicator bacteria can.
Furthermore, when environmental conditions make it really difficult for them to grow, enterococci and many other bacteria can enter a state in which they are alive but unable to form colonies, thus avoiding standard methods of detection. This doesn’t stop them from springing back to their old disease-causing selves, when the conditions are right.
Improved methods on the horizon
We know some things about how these bacteria operate in the ocean, but if we knew more, we could design more thorough and faster sampling and detection methods that would take this knowledge into account. Some of the most promising newer methods are based on quantifying the amount of the indicator organism’s DNA in the water or sand. DNA-based methods can be completed quickly (four to six hours), which would shorten the lag in decision-making. These methods also tally other indicator organisms that might not form colonies.
In the lab of Rebecca Gast, a microbial ecologist at Woods Hole Oceanographic Institution, we’re using DNA-based molecular methods to count indicator organisms in beach sands and see how their numbers compare at different parts of beaches and over the course of the summer. Other new insights could come from frequent automatic sampling systems, which would provide basic information on how quickly populations of indicator organisms change in water, as well as insights on the environmental conditions that allow them to thrive. Chris Scholin, a scientist at the Monterey Bay Aquarium Research Institute, has developed one of these, called the Environmental Sample Processor (“ESP”).
Because the nature of the problem is ultimately tied to coastal development and use—more roads and more parking lots, more people, and more sewage overflow—the methods we devise to understand bacterial water quality may also help us find better ways to avoid or lessen human impacts on coastlines.
In the end, protecting the quality of coastal waters translates to enjoying local beaches on the spur of the moment, without second guesses about what might be found there tomorrow, or the next day.
Halliday’s research is funded by the National Science Foundation and the Woods Hole Center for Oceans and Human Health, and is in collaboration with the Southern California Coastal Water Research Program. She is also supported by the Stanley W. Watson Student Fellowship Fund at WHOI and The J. Seward Johnson Fund at WHOI. This article was written during a science writing course for graduate students at WHOI, supported by funds from The Henry L. and Grace Doherty Professor of Oceanography.
From the Series
Slideshow
Slideshow
- To test water safety at swimming beaches, workers take water samples, filter them to collect the bacterial cells, and place them on a culture medium that promotes the growth of only enterococcus bacteria—an indicator of contamination. Single cells can grow explosivley into colonies (blue because of a pH-indicator dye in the medium). Massachusetts bathing beach regulations set the limit for enterococcus cells in beach waters at 104 "colony forming units" in 100 millimeters. (Courtesy of Bethany Sadlowski, Barnstable County Department of Health and Environment)
- Regular testing leads to beach closures if high levels of bacteria are found in the water. But the time needed to grow and count bacterial colonies inserts a lag time of days between sampling and when signs are posted. Beaches may be closed after bacteria have dispersed, or kept open when bacteria are present but not yet counted. New methods of detection based on identifying bacterial DNA one day may significantly shorten the time between sampling and results and let managers make more timely decisions about beaches. (Courtesy of Elizabeth Halliday, Woods Hole Oceanographic Institution)
- Elizabeth Halliday, a graduate student in the MIT'WHOI Joint Program, takes samples of sand at Wood Neck Beach in Falmouth, Mass., to analyze in the laboratory. Bacteria that are indicators of fecal contamination can live and grow in beach sand, and the DNA from microbes living within this sand will be used to find out how many indicator bacteria are present. (Courtesy of Elizabeth Halliday, Woods Hole Oceanographic Institution)
Related Articles
- The ocean currents behind Brazil’s pollution problem
- How is human health impacted by marine plastics?
- Sunlight and the fate of oil at sea
- A toxic double whammy for sea anemones
- WHOI scientists discuss the chemistry behind Sri Lanka’s flaming plastic spill
- Oil spill response beneath the ice
- Fukushima and the Ocean: A decade of disaster response
- WHOI scientist shares her perspective on ‘imminent’ oil spill in the Red Sea
- WHOI establishes new fund to accelerate microplastics innovation


