What Happened to Deepwater Horizon Oil?
Did dispersants help microbes break down oil?
Six years after the Deepwater Horizon oil spill, we are continually asked two questions. What happened to the 160 million gallons of oil that gushed for 87 days into the Gulf of Mexico in 2010? Was discharging 1.67 million gallons of chemicals into the ocean to disperse the oil a good or bad idea?
In a study we published this week in the Proceedings of the National Academy of Sciences (PNAS), we offer evidence to answer those questions.
From a scientist’s point of view, the Gulf of Mexico is a vast beaker and the Deepwater Horizon disaster represented the forbidden experiment that we would never purposely conduct but which we nevertheless take advantage of to learn lessons that we can apply to future oil spills.
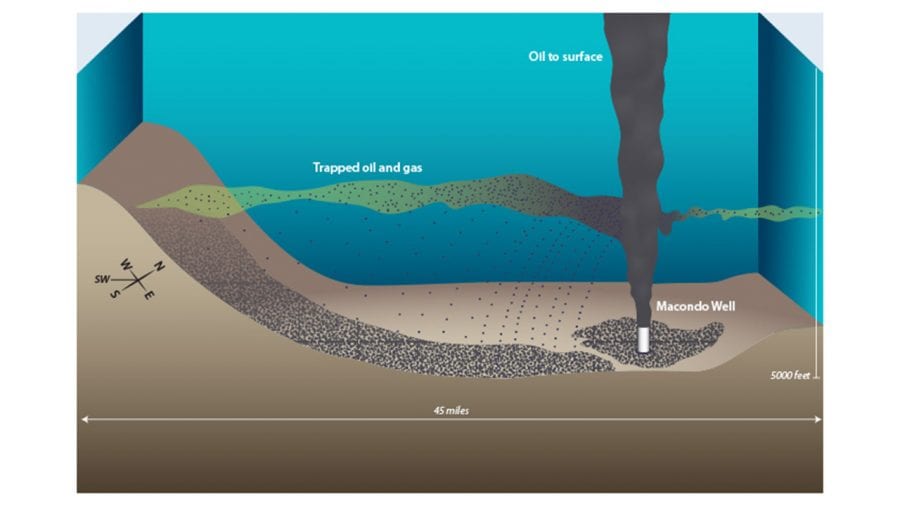
In 2014 we published a study in PNAS that partially answered the first question. We identified how much and where Deepwater Horizon oil was deposited onto the deep seafloor. In our new study, we examined more than 400 sediment seafloor samples and investigated what happened to that oil in the cold ocean depths in the four years after the spill.
The fate of spilled oil
Oil isn’t homogeneous, but a mixture of numerous individual hydrocarbon compounds. So we investigated the fate of 125 distinct chemical compounds, each with different structures and properties. Those compounds represent mounds of food at a buffet table for marine microbes.
Like people, microbes can be selective about which food they choose to eat. In laboratory experiments and past oil spills near the ocean surface, we’ve seen that microbes prefer to chow down on smaller hydrocarbon compounds—ones that have simple linear structures, as opposed to ones with long branches or ring structures. Microbes typically leave behind the larger, more complex compounds that require more effort to break down. We expected these patterns might be accentuated at great ocean depth where colder temperatures tend to slow down metabolic rates.
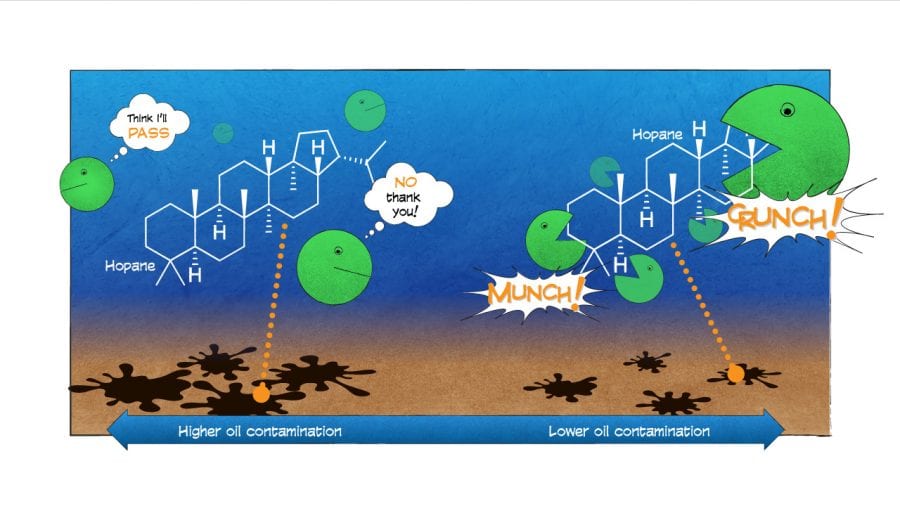
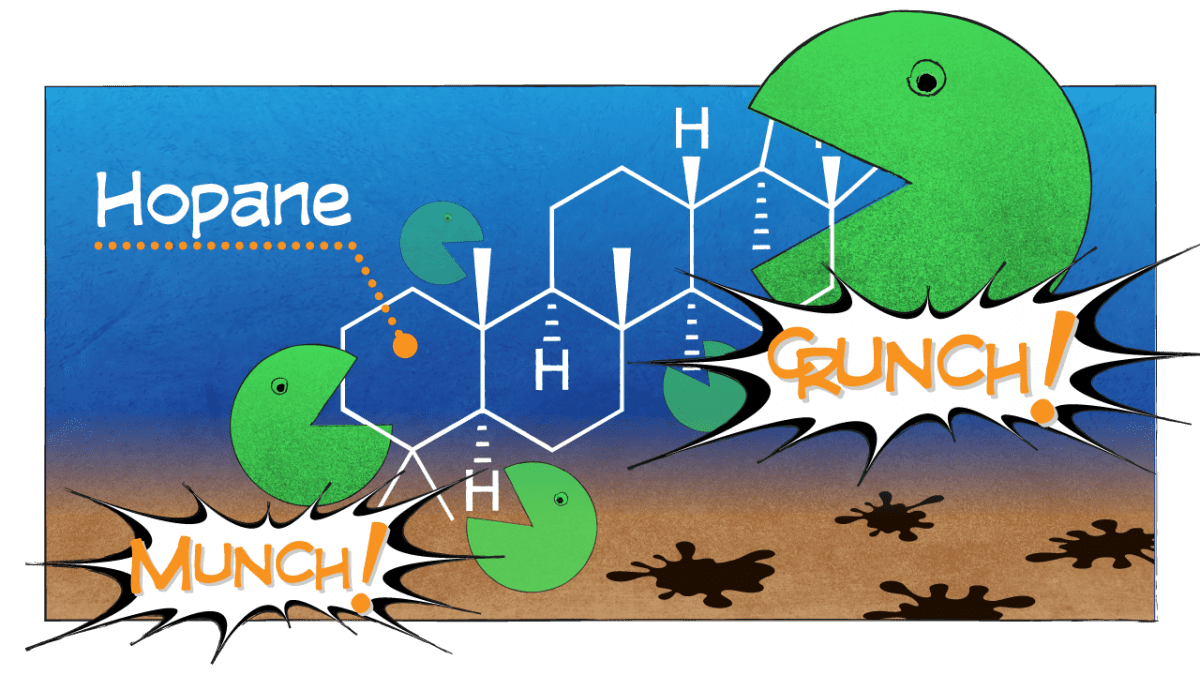
In general, we found that compounds with simple structures were eaten first. But we were surprised to find that in the frigid abyss, microbes also decomposed a complex compound with many branches and rings called hopane.
Hopane is generally so resistant to degradation that oil-spill scientists have come to rely on it as a reference to contrast the relative degradation of all other compounds and to help “fingerprint” the source of where oil came from. Our finding throws a curveball to oil-spill scientists: We can no longer assume that hopane and other complex hydrocarbons don’t break down in the environment.
A controversial decision
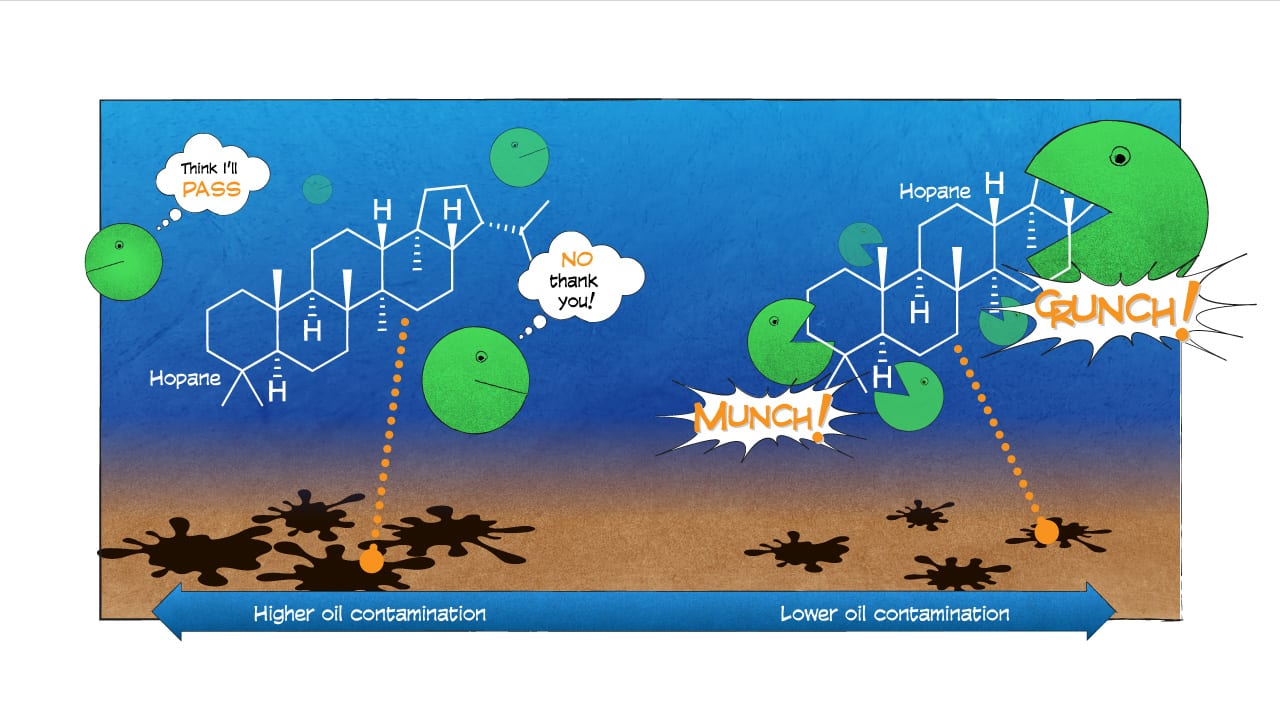
Our new study, whose lead author was Sarah Bagby, a postdoctoral scientist at the University of California, Santa Barbara, also revealed that hopane didn’t uniformly degrade in the environment. It didn’t degrade in seafloor sediments that were heavily contaminated with oil residues. It did decompose, however, in areas less contaminated by oil.
This pattern possibly was related to how much surface area of the oil was exposed to would-be microbial consumers. In places where hopane was deposited in large clumps covered by sediments, microbes had fewer points of attack. But where small droplets of oil were suspended in the water, microbes could get to the hopane more easily and break it down. Even the undercooked broccoli will get taken from the buffet if it’s placed up front and easy to reach.
This observation leads to the question about the decision to use dispersants. Officials released 900,000 gallons of dispersants at the ocean surface to break down oil into smaller, non-clumping droplets in order to reduce the impacts of would-be oil slicks. That is standard protocol for oil spills. But the Deepwater Horizon spill occurred at unprecedented depths in the ocean, and in this case, officials took the unprecedented and controversial step of also pumping 771,000 gallons of dispersants at the seafloor.
It seems counterintuitive that adding unnatural chemicals to the environment could be beneficial. On the other hand, we do it routinely to combat perceived larger threats, such as dropping flame retardants to control wildfires.
People on either side of the issue seem convinced that dispersants are either an extremely useful or dangerous tool, but there has been little field-based evidence to support either view. That’s because studying oil spills is hard, especially in their earliest stages, when research reasonably takes a back seat to the more pressing need to save lives and prevent damage. Studying a spill during an active response is akin to asking firefighters to step aside, so we can sample some smoke or water from the burning home. But once the fire is out, crucial evidence may be lost and only forensics remain.
Informed decisions
In the midst of the Deepwater Horizon disaster, officials debated whether to introduce a control into the “experiment” by shutting down the use of dispersants for a time long enough to see if it made any difference. But those in command decided to keep the dispersants flowing, so we must turn to environmental forensics to reconstruct what happened.
Our research examining patterns of oil degradation uncovered evidence that microbes ate oil more readily when it came in smaller droplets suspended in water—before it clumped with other material and settled to the seafloor. That’s just what dispersants do: They break up oil into smaller drops so that they don’t readily clump together, float or settle, but rather remain suspended longer and travel farther in the ocean, exposing more surface area for longer time periods to microbial attack.
In short, we have compelling circumstantial evidence that dispersants may have boosted the activity of microbes and the breakdown of spilled oil. This, of course, must be balanced with the potential harmful impacts to the environment caused by prolonged exposure to the dispersant or dispersed oil.
Compared with imperfect laboratory experiments to simulate what happened, this is a rare finding from the field of what actually happened. It’s useful information, but will officials consider it when faced with a decision on whether or not to use dispersants during the next oil spill?
Recognizing the controversy raised by proponents and opponents of using dispersants, the National Academy of Sciences is planning to perform an objective, fact-based, science-rich review of the data available on dispersants. As scientists, we don’t come down on either side of the issue. Our job is to investigate and compile the facts and actively communicate them to policymakers—so that they can make informed, and therefore wiser, decisions.
This research was funded by the National Science Foundation and the Simons Foundation.
From the Series
Slideshow
Slideshow
- Hopane, a component of oil, is a large, complex compound with many branches and rings. Microbes typically leave behind larger, more complex compounds that require more effort to break down. In a new study, scientists were surprised to find that in the frigid abyss on the seafloor of the Gulf of Mexico, microbes did decompose hopane. Hopane is generally so resistant to degradation that oil-spill scientists have come to rely on it as a reference against which to measure the relative degradation of all other compounds, and to help “fingerprint” the source of where oil came from. The new finding throws a curveball to oil-spill scientists, who can no longer assume that hopane and other complex hydrocarbons don’t break down in the environment. (Illustration by Eric S. Taylor, WHOI Graphic Services)
- A new study on what happened to oil spilled during the Deepwater Horizon disaster suggests that microbes may be able to degrade small oil particles more rapidly than large ones, because small particles have more exposed surface area. The evidence suggests that the subsurface release of dispersants, which keep oil droplets from clumping together, may have boosted the activity of microbes and the breakdown of spilled oil. (Illustration by Eric S. Taylor, WHOI Graphic Services)
- Oil and gas were trapped in a plume that flowed at depth from the damaged Macondo well in the Deepwater Horizon disaster in the Gulf of Mexico. In a 2014 study, scientists proposed that tiny droplets of oil in the plume coagulated and sank to the seafloor—much of it within 45 miles of the well. (Illustration by Jack Cook, WHOI Graphic Services)
- A remotely operated deep-sea vehicle is loaded with plastic cylinders to be used as push-cores to take samples of seafloor sediments contaminated by the Deepwater Horizon oil spill in the Gulf of Mexico. (Photo by David Valentine, University of California, Santa Barbara)
- Some 160 million gallons of oil gushed for 87 days into the Gulf of Mexico during the Deepwater Horizon disaster in 2010. Officials also discharged 1.67 million gallons of chemicals into the ocean to disperse the oil. (Photo by Dan Torres, Woods Hole Oceanographic Institution)
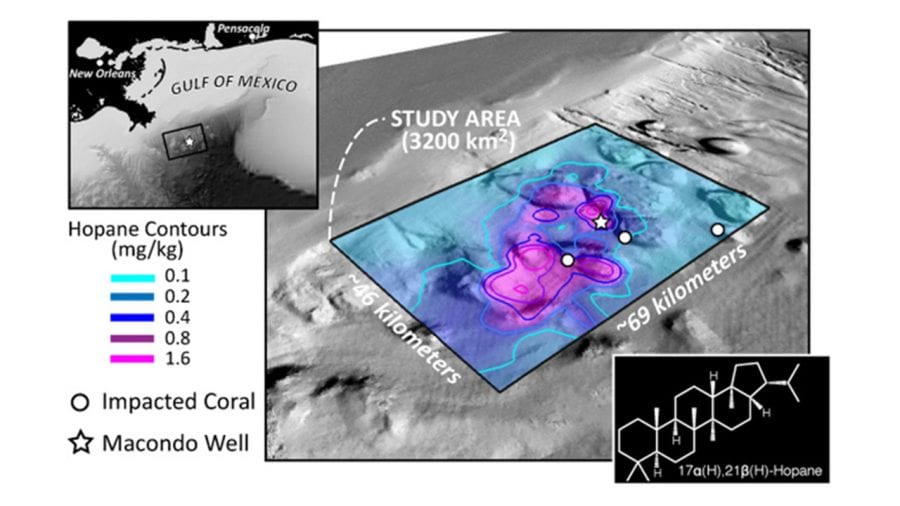
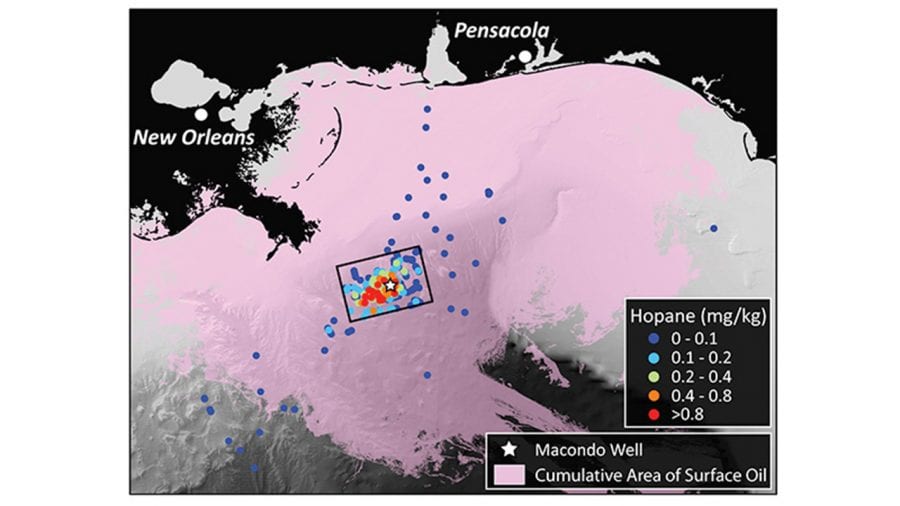
- In the Deepwater Horizon disaster, oil from the damaged Macondo well on the seafloor rose and formed slicks at the surface that spread far and wide (lavender), driven by winds and currents in the Gulf of Mexico. But some portion of the oil was trapped on the bottom and never rose to the surface. Scientists studied samples of seafloor sediments (dots) and focused on a telltale chemical, hopane, to determine that the trapped oil settled in a relatively small area near the well (box). (Image courtesy of G. Burch Fisher, University of California, Santa Barbara)
- Scientists found elevated levels of hopane from Deepwater Horizon oil within a 1,250-square-mile box near the damaged well (star) and near communities of deep-sea corals damaged by the oil spill (circles). They found that 4 to 31 percent of the oil trapped in the deep ocean—the equivalent of 2 to 16 percent of the total oil discharged during the accident—fell within that 1,250-square-mile patch. (Image courtesy of David L. Valentine, University of California, Santa Barbara)
- This article's authors, marine chemists Chris Reddy (left) of the Woods Hole Oceanographic Institution and David Valentine of the University of California, Santa Barbara, flew above the Gulf of Mexico to observe the aftermath of the Deepwater Horizon oil spill. (Photo courtesy of Chris Reddy, Woods Hole Oceanographic Institution)
Related Articles
- 4 Potential Solutions for Corals in Crisis
- An ocean of opportunity
- Putting the ‘nuclear coffin’ in perspective
- Chasing Ocean ‘Snowflakes’
- Journey to the Bottom of the Sea
- Life at the Edge
- The Discovery of Hydrothermal Vents
- Mission to the Ocean Twilight Zone
- New Device Reveals What Ocean Microbes Do
See Also
- Persistence and biodegradation of oil at the ocean floor following Deepwater Horizon from the Proceedings of the Natinal Acaemy of Sciences
- Where Did the Deepwater Horizon Oil Go?
- David Valentine's Lab at UCSB
- Chris Reddy, Woods Hole Oceanographic Institution
- Deepwater Horizon Aftermath UCSB news release
- Oceanus Special Issue on Deepwater Horizon
- Science in a Time of Crisis WHOI's response to the Deepwater Horizon Oil Spill
- Oil in the Ocean