
Mistaken Identity
Two bromine compounds found in whale blubber are natural products, not industrial pollutants
Researchers at the Woods Hole Oceanographic Institution have found that two chemicals accumulating in the tissues of marine animals and suspected to be manmade pollutants actually came from natural sources.
Brominated organic chemicals are used as flame retardants for electronics, furniture, and textiles. In recent years, they have been found throughout the environment, accumulating in fish and marine animals and sometimes detected in human breast milk. Some researchers suspect that these compounds may affect animal and human health, and several compounds have been banned.
By isolating 1 milligram of (MeO-BDEs) from 10 kilograms (22 pounds) of whale blubber, Emma Teuten, a postdoctoral fellow in the WHOI Department of Marine Chemistry and Geochemistry (MCG), found that the MeO-BDEs in the blubber contained carbon-14 (14C). This natural radioactive isotope of carbon is incorporated into all living things, but it would have long ago decayed out of the petrochemicals used by industrial chemists. That means Teuten’s suspect chemicals were derived from a natural, though still unidentified, source.
“The work shows that natural products with similar structures to industrial compounds can also bioaccumulate,” said Teuten. “Consequently, animals have been exposed to them for many years. The presence of natural analogs may help toxicologists explain how and why enzymes have the ability to metabolize industrial halogenated compounds, such as polychlorinated biphenyls (PCBs).”
The finding was published Feb. 11 in the journal Science. The work was performed in the laboratory of Associate Scientist Chris Reddy (MCG), who was a key contributor to the work along with Geology and Geophysics Research Associate Li Xu.
“The accumulation of human-made chemicals such as PCBs and polybrominated diphenyl ethers in whales and other top consumers has been known for decades,” said Mark Hahn, a marine toxicologist in the WHOI Biology Department. “The difficulty is that some anthropogenic compounds are also made naturally by some organisms, and some anthropogenic compounds can be biologically or chemically transformed in the environment to derivatives that resemble natural products.”
“Teuten and Reddy have provided the first definitive evidence that specific BDE derivatives are in fact naturally produced,” Hahn added. “They did this by combining an elegant analytical method—analysis of radiocarbon content—with the brute force purification of the compounds from large amounts of whale blubber.”
Wanted: dead or alive
It took 18 months, three blenders, and dozens of dulled knives to conduct this experiment.
Proving the origin of the MeO-BDEs seems like a simple problem: Find 14C in a sample, and you know whether the chemicals came from the factory or Mother Nature.
“In the laboratory, we call this approach the ‘dead or alive theory,’” said Reddy. “Petrochemicals are radiocarbon-dead and natural products are radiocarbon-alive.”
But to detect 14C, chemists require a sizable sample. To detect 14C within the scarce molecules of brominated compounds required a very large sample.
The first challenge was acquiring a large piece of whale blubber. Reddy requested and received a permit from the National Marine Fisheries Service, which limits the “take” of marine mammals for research. Teuten then contacted researchers at various marine mammal stranding and rescue operations to alert them that they needed a sample the next time one was available.
A new tool and a telltale clue
By the fall of 2003, the unfortunate beaching and death of a True’s beaked whale in Virginia turned into good fortune for the WHOI research team. Teuten received a package from the Virginia Marine Science Museum containing 10 kilograms of foul-smelling but scientifically precious whale flesh.
That’s when the brute force portion of the experiment began. Teuten had to chop, cube, and blend mounds of whale blubber, a task made even less appealing by the fact that she is a vegetarian.
“It was messy, oily work, and I never thought working with blubber would be so nasty,” Teuten said. She dulled many a knife and had to purchase three blenders before moving on to more traditional tools of chemistry, such as filtering, acid washes, dialysis techniques, and chromatography.
“What Emma did was heroic, like finding a needle in a haystack,” said Reddy. “She removed 10,000,000 milligrams of other whale material—mainly fats—to get our compounds of interest in very high purity.”
“There was no road map for this,” Teuten said. Most chemical extractions involve work with 50 grams; Teuten started with 200 times that in order to isolate just 1 milligram of the brominated compounds. “This kind of thing just hasn’t been done much in radiocarbon analysis.”
Teuten and Reddy finally submitted their sample of brominated compounds to the National Ocean Sciences Accelerator Mass Spectrometer facility (based at WHOI) for analysis, where chromatography expert Li Xu became involved in the effort. They found 14C.
“This radiocarbon technique is very exciting,” said Gordon Gribble, an organic chemistry professor at Dartmouth College and a leader in the study of halogenated compounds found naturally in the environment. “There’s been no other way to distinguish the origin of the same compounds that are produced both by nature and man.”
Chemicals are everywhere, and they’re not all bad
In recent years, polybrominated diphenyl ethers (PBDEs) have been found in freshwater fish near industrialized areas of the Great Lakes and Northern Europe, though there are no known natural sources for these chemicals in fresh water. But natural PBDEs also have been found in sea sponges off Australia and in the dolphins living nearby.
Based on preliminary findings of possible health risks linked to PBDEs, the European Union and the state of California have banned certain formulations of flame retardants. Industrial producers counter that the compounds are non-reactive, do not degrade in the environment, and therefore are safe. But no one really knows for sure.
For decades, environmental groups have said that nature would never make brominated compounds or other halogenated chemicals, Gribble noted. But in recent years, these compounds have been found in forest fires, volcanic ash, soil, peat bogs, and myriad marine organisms.
“It appears that nature has been producing these chemicals since the first forest fire and since life on Earth began,” he said. In the ocean, where many of these compounds are ubiquitous, organisms have developed ways of synthesizing these compounds from oceanic salt for use as natural pesticides and repellents.
“As we design environmental laws for the regulation of chemicals, we have to be aware of what nature is making,” said Gribble. “We have to evaluate each chemical on a case-by-case basis.”
“Many people have the simplistic idea that synthetic equals bad and natural equals good,” said Hahn. “Teuten and Reddy’s work shows that naturally produced compounds can bioaccumulate to levels similar to those of some notorious contaminants. Whether these natural compounds are affecting the health of the whales or other animals that accumulate them is an open question that needs to be addressed.”
“These sort of compounds are out there, globally distributed, accumulating in wildlife and human tissues,” Hahn added. “We are still making some of them, and our decisions about whether to continue or whether to substitute other compounds should be based on knowledge about the amounts and effects of both naturally produced and anthropogenic compounds.”
The study was supported by the National Science Foundation, The Camille and Henry Dreyfus Foundation, Inc., the J. Seward Johnson Fund, and the WHOI Ocean Life Institute.
Slideshow
Slideshow
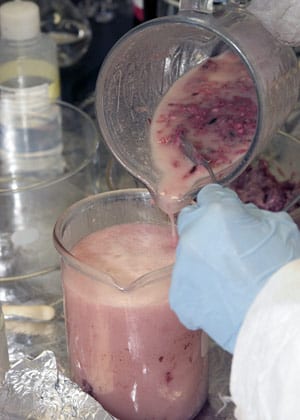
- Chemist Emma Teuten tries to strain and hold back large, grainy pieces of blubber while pouring her blended mixture into a beaker. (Photo by Tom Kleindinst, WHOI Graphic Services)
- A vacuum is used to suck fats (lipids) out of the pink, blended whale blubber (beaker at the top) through a filter and into a flask (filled with gold liquid). In the background, chopped chunks of blubber await their trip in the blender. (Photo by Tom Kleindinst, WHOI Graphic Services)
- Emma Teuten pours concentrated sulfuric acid into flask full of fat-filled whale extract, turning the mixture black. (Photo by Tom Kleindinst, WHOI Graphic Services)
- After concentrated sulfuric acid was mixed into the whale blubber fluids (producing a black, viscous mixture), Teuten added hexane to help separate the needed halogenated compounds from the mix of fat and acid. In the photo, the golden liquid in the top flask is the hexane-halogen mixture, while the blackened mix of lipids and acid filter through a seperatory funnel (middle) into a beaker on the bottom. (Photo by Tom Kleindinst, WHOI Graphic Services)
- Teuten washes the hexane extract with water to remove the remaining acid (foreground), then passes it through a silica gel (white mixture in column) to further purify the sample down to the chemicals that she needs to study. (Photo by Tom Kleindinst, WHOI Graphic Services)
- Teuten uses a rotary evaporator to remove excess solvent from her sample. (Photo by Tom Kleindinst, WHOI Graphic Services)
- The whale extract is passed through silica gel (grey-white fluid in the column near the top of the photo) to separate the last of the lipids out of the sample. The black material near the top is lipid, while the clear fluid in the bottom flask is the extract of methoxy and halogenated compounds that Teuten was working to isolate. (Photo by Tom Kleindinst, WHOI Graphic Services)
- Teuten (left) and Li Xu examine preliminary results from the gas chromatograph in the National Ocean Sciences Accelerator Mass Spectrometer facility. Modern equipment allows them to isolate and collect data on the individual chemical compounds in a sample. (Photo by Tom Kleindinst, WHOI Graphic Services)
Related Articles
Topics
See Also
- WHOI Press Release
- WHOI Department of Marine Chemistry and Geochemistry
- National Ocean Sciences Accelerator Mass Spectrometer
- Mark Hahn's Laboratory
- Gordon Gribble's Research Page
- Science News: Flaming Out? Days may be numbered for two fire retardants
- Environmental Science and Technology: What Fate for Brominated Fire Retardants?







