
How Does Nature Deal with Persistent Pollutants?
Graduate student explores biomagnification of chemicals up the food chain
Why would I choose to spend my years in graduate school up to my elbows in foul-smelling whale blubber? To explore how some of the most notorious man-made pollutants reach dangerous concentrations in large predators, even when concentrations of these pollutants in seawater are low and considered “safe.”
When I entered graduate school I knew that some man-made pollutants could be concentrated in large predators. I had heard how DDT caused the collapse of bald eagle populations, and I was aware that eating a lot of swordfish was a bad idea because of mercury contamination. But I was surprised and intrigued when my Ph.D advisor, Christopher Reddy, explained that researchers have recently discovered a few naturally produced chemicals that also accumulate with each link up the food chain—a process called biomagnification.
These natural biomagnifying compounds have existed in the environment for eons, far longer than their man-made counterparts, with no apparent harm to the environment. Ecosystems have evolved with these compounds and seem to be able to deal with their presence. So, if we can better understand how naturally produced biomagnifying compounds travel through—and eventually exit—ecosystems, we can better predict the pathways and fates of man-made biomagnifying pollutants now littering the oceans.
When I explained my research to my father, he had his own take on it: “Very interesting theory,” he said, “Learning from Mother Nature to help clean up the kids’ messes.”
The ins and outs of ecosystems
Biomagnification occurs when contaminants that don’t easily degrade increase with each link of a food chain. And they don’t go away—they can stick around for decades, and maybe even centuries. That is why most of the world has banned or restricted their use.
Here is what happens. In seawater these persistent molecules stick to small particles and phytoplankton. Small fish eat the phytoplankton, but the contaminants can’t be broken down and are absorbed, intact, by the fish. When small fish are eaten by larger predators, the process repeats—again and again, up the food chain. Each subsequent predator receives a higher dose than the previous one. Animals at the top of the food chain, such as dolphins, receive the most concentrated dose of these contaminants with every meal.
Biomagnification has been well known among some man-made pollutants but not for natural products. Until Chris introduced me to biomagnifying natural compounds, natural compounds had all sorts of good connotations in my mind: therapeutic, gentle, miracle drugs. But I should not have been surprised to have this worldview overturned. After all, brevetoxin, the naturally produced red tide poison, graces the cover of my college organic chemistry textbook.
But even after grappling with the concept of potentially harmful natural products, I was still captivated by the idea that some natural products could biomagnify. Why? Basically, because nature usually balances its checkbook. Whatever enters an ecosystem eventually gets processed back out. Over time, the incoming and outgoing amounts should be equal.
The Cape Cod Stranding Network
Studying these long-lived natural products requires analyzing marine animals. To make my job easier, I start with samples rich in biomagnified contaminants—blubber!
But how do you get your hands on pounds and pounds of blubber? Whales, dolphins and seals are protected; we can’t hunt them or take their lives in the name of science. As a child, I was enthralled by these magnificent animals, and I still am. I wouldn’t want to harm them for any purpose, scientific or otherwise.
Fortunately, my lab works with the Cape Cod Stranding Network (CCSN), which strives to help the hundreds of animals stranded each year on the beaches of Cape Cod. Rescuers push some back out to sea, and bring others into aquariums for rehabilitation and release. But, unfortunately, some animals just don’t make it.
The CCSN ensures that these losses benefit living animals by performing necropsies (the animal equivalent of autopsies) and distributing tissues to researchers for studies on diseases, boat/motor-collisions, and, for me, chemical analyses.
Make mine a whale smoothie
From the large slabs of frozen blubber that I receive from the CCSN, I make a viscous, brilliantly yellow, and very fragrant oil. Unlike the whalers of previous generations, I do not “cook” the oil out of the blubber, because that might alter its chemical signature. Instead, I use a blender to make a blubber “smoothie.”
It is really quite gross. However, simply by filtering this pungent “smoothie,” I end up with clear, banana-yellow oil that those long-ago whalers would envy.
All the compounds that I analyze are floating around in this oil. To separate them from the oil, I take advantage of the size difference between the oil molecules, which are very large, and my compounds, which are very small.
I pass the oil through a long glass column packed with particles that have tiny cavities. The small molecules go in and out of these cavities. They take their time and pass through every nook and cranny. The larger oil molecules can’t fit in the crevices, so the bright yellow oil rushes straight through. I discard the oil and collect the interesting molecules all by themselves.
I still need to purify my extract further. I repeat the process using different columns that separate molecules by different properties, such as volatility (how easily they escape into the air) or polarity (how electrons are arranged in a molecule). As molecules emerge from the final column, I can determine their identities and how much was present in the original blubber.
Natural cousins of pollutants
In my four years as a graduate student, I’ve established that a new class of natural compounds is widespread in the blubber and livers of marine mammals in the North Atlantic. The impossible-to-pronounce name for these chemicals is the halogenated 1’methyl-1,2’-bipyrroles, commonly abbreviated MBPs.
We don’t yet know where MBPs come from, what function they might provide, or why they accumulate in marine mammals. But we can tell that MBPs are remarkably similar to many man-made pollutants that biomagnify. Both turn up in similar amounts in my blubber and liver samples. This suggests that MBPs biomagnify as well.
To confirm that MBPs biomagnify, my next step is to analyze lots of fish, squid, crustaceans and plankton from the Northwestern Atlantic—creatures representing other links within this food chain. By measuring MBP concentrations in these samples, I’ll see if MBPs really do magnify at each level.
Now, I just have to get my hands on lots of fish. As far as I know, there is no “Fish Stranding Network.” But the National Marine Fisheries is just down the road from my lab, and I hear they take requests.
Kristin Pangallo’s research was funded by the National Science Foundation, The Seaver Institute, the J. Seward Johnson Fund, and The Virginia Walker Smith Fund. This article was written during a science writing course for graduate students at WHOI, supported by funds from The Henry L. and Grace Doherty Professor of Oceanography.
From the Series
Slideshow
Slideshow
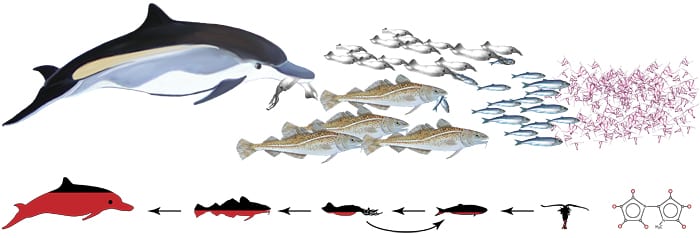
 Biomagnification occurs when contaminants that don’t easily degrade increase with each link of a food chain. In seawater, these persistent molecules stick to small particles and phytoplankton. Small fish eat the phytoplankton, but the contaminants can’t be broken down and are absorbed, intact, by the fish. When small fish are eaten by larger predators, the process repeats—again and again, up the food chain. Each subsequent predator receives a higher dose than the previous one. Animals at the top of the food chain, such as dolphins, receive the most concentrated dose of these contaminants with every meal. MIT/WHOI graduate student Kristin Pangallo is studying naturally produced, persistent molecules—a halogenated 1' -methyl-1,2' -bipyrrole, or MBP (above)—found in marine mammal blubbler to help learn more about what happens to man-made persistent chemicals in the environment. (Illustration by E. Paul Oberlander, Woods Hole Oceanographic Institution)
Biomagnification occurs when contaminants that don’t easily degrade increase with each link of a food chain. In seawater, these persistent molecules stick to small particles and phytoplankton. Small fish eat the phytoplankton, but the contaminants can’t be broken down and are absorbed, intact, by the fish. When small fish are eaten by larger predators, the process repeats—again and again, up the food chain. Each subsequent predator receives a higher dose than the previous one. Animals at the top of the food chain, such as dolphins, receive the most concentrated dose of these contaminants with every meal. MIT/WHOI graduate student Kristin Pangallo is studying naturally produced, persistent molecules—a halogenated 1' -methyl-1,2' -bipyrrole, or MBP (above)—found in marine mammal blubbler to help learn more about what happens to man-made persistent chemicals in the environment. (Illustration by E. Paul Oberlander, Woods Hole Oceanographic Institution)- >MIT/WHOI graduate student Kristin Pangallo started her research with blubber saved from whales that died after being stranded on beaches. She puts the blubber through an elaborate process to produce samples of whale extract Subsequently, she has examined animals further down the food chain. Above is a flask of squid extract. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
- MIT/WHOI graduate student Kristin Pangallo then purifies her marine animal extracts further to extract biomagnifiying chemicals, including naturally occuring ones that have existed for eons. Those chemicals could help us learn how to deal with more recently introduced man-made pollutants that are accumulating in marine life. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
Related Articles
- The ocean currents behind Brazil’s pollution problem
- How is human health impacted by marine plastics?
- Sunlight and the fate of oil at sea
- A toxic double whammy for sea anemones
- WHOI scientists discuss the chemistry behind Sri Lanka’s flaming plastic spill
- Oil spill response beneath the ice
- Fukushima and the Ocean: A decade of disaster response
- WHOI scientist shares her perspective on ‘imminent’ oil spill in the Red Sea
- WHOI establishes new fund to accelerate microplastics innovation
Topics
Featured Researchers
See Also
- Mistaken Identity Two bromine compounds found in whale blubber are natural products, not industrial pollutants (from Oceanus magazine)


