Cell-sized Thermometers
Can the shells of microscopic organisms be used to measure past deep-ocean temperatures?
Climate shifts are a repeating feature in Earth’s history, but humans have added so much greenhouse gas (especially carbon dioxide) to the atmosphere that climate is warming in our lifetimes. We know that past climate changes have concurred with changes in the circulation and temperatures of the deep ocean. So understanding how and why deep-sea temperatures changed could help us understand past climate changes and predict future changes. But estimating the past temperature of the deep ocean has to rely on proxy measurements, not direct ones.
Fortunately, nature has dropped a recording thermometer into our hands. Scientists have discovered that the chemical composition of shells of marine organisms hold and preserve clues to past water temperatures: The composition is different in shells forming at different temperatures. Climate scientists now widely use aspects of shell composition as proxies for past temperatures.
The fossil shells of single-celled organisms called foraminifera are the best means we have of looking at the deep ocean over the past 20,000 years. Some foraminifera live abundantly in the upper ocean and their shells rain down to become seafloor sediment, but other foraminiferal species (“benthic” foraminifera) live on and in the seafloor, where their shell compositions record the temperature of the deep sea.
The shells become buried in seafloor sediments that accumulate over time. Deeper sediment layers were deposited further back in time. Scientists can analyze these shells to estimate deep-sea temperatures back to the last ice age, through times of great climate variability.
A new culture system
Here’s how the thermometer works. Many marine organisms, including clams, corals, and some foraminifera, make hard shells or skeletons of calcium carbonate—the same mineral as chalk or marble. Seawater contains abundant calcium and dissolved carbon dioxide (or carbonate). It also contains trace elements including magnesium and strontium, and stable isotopes of carbon and oxygen. These trace substances are incorporated into the shell structure, in amounts that vary with water temperature. The amount of magnesium provides the proxy we use to estimate deep-sea temperatures: A higher ratio of magnesium to calcium in shells of deep-living foraminifera means they formed at a higher temperature.
The question is: Is the thermometer accurate? To trust a thermometer’s readings, we must first calibrate the thermometer, by verifying that a certain amount of change on the thermometer’s scale is reliably equivalent to a degree of temperature change.
So we are trying to calibrate this benthic thermometer in the laboratory for the first time, by culturing benthic foraminifera in our lab and studying their shell composition under tightly controlled physical and chemical conditions. We built a culture system with commercial-grade refrigerators, re-circulating seawater, and small chambers, or “microcosms,” that house only a few cells each. What we need now is a green thumb, and some luck.
Challenging culture conditions
On a cruise aboard Woods Hole Oceanographic Institution’s research vessel Oceanus in May, 2006, we collected live foraminifera from the top layer of deep-sea sediments off the Bahamas. We kept the samples at cold, deep-sea temperatures (though not at high pressure), and brought them back to culture.
To examine only one variable at a time, we at WHOI are growing deep-sea foraminifera at multiple temperatures, while we carefully control the concentrations of carbonate ions, trace elements, and acidity, for example—to keep the physical and chemical environment constant. The WHOI Ocean and Climate Change Institute is funding this research.
If the foraminifera grow, thrive, add new shell material and/or reproduce at the selected temperatures, we will analyze the magnesium-to-calcium ratios of the new calcite, using the Cameca IMS 3F secondary ion mass spectrometry (SIMS) system at WHOI, and determine their relationship to temperature.
Meanwhile, we, with our colleagues Tom Chandler, Tim Shaw, and Chris Hintz at the University of South Carolina, are doing a complementary study, holding temperature constant and varying the seawater chemistry to find its relationship to foraminiferal shell composition.
Other factors—including the cell’s age, for example—may also influence shell composition and confuse the temperature reading. And the top layer of seafloor sediments can be mixed by currents or burrowing animal activity, upsetting the sediments’ layered sequence and inferred chronology and age. So it’s even more important to define the relationship between seawater chemistry, temperature, and shell composition using our well-constrained culturing approaches. Our efforts, we hope, will provide a dependable and reliable calibration that researchers can use to better understand how deep-sea temperatures reacted during past climate fluctuations.
The 2006 cruise to collect benthic foraminifera was supported by the National Science Foundation Ocean Sciences Marine Geology and Geophysics Program.
Slideshow

Slideshow
- Working in the cold lab aboard the WHOI research vessel Oceanus, biogeochemist Joan Bernhard removes subcores containing deep-sea single-celled organisms from a core of seafloor sediment. (Photo by Joanne Eberhardt, Pratt Institute)
- Inside out: WHOI geochemist Dan McCorkle, bundled up for the cold lab in spite of the ship's balmy location off the Bahamas, works with deep-sea sediment samples containing the single-celled bottom-living organisms (foraminifera) he and Joan Bernhard are looking for. (Photo by Joanne Eberhardt, Pratt Institute)
- Outside on Oceanus's deck, Dan McCorkle (left) and Joan Berhnard maneuver into place the deep-sea multiple corer. (Photo by Joanne Eberhardt, Pratt Institute)
- Joan Bernhard (green hard hat), with University of South Carolina student Katrina Phillips on board R/V Oceanus, carefully carries a sediment core into a refrigerated lab van. (Photo by Joanne Eberhardt, Pratt Institute)
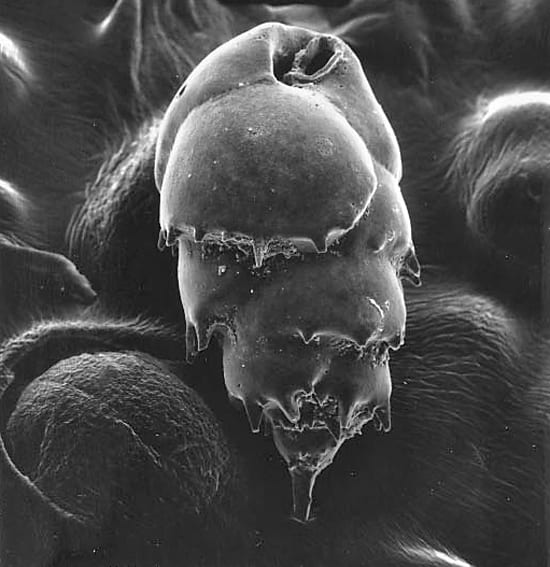
- Some benthic foraminifera, such as the one shown in this scanning electron micrograph, are single-celled organisms whose calcium carbonate shells contain information about the temperature of the water they grew in. This may allow them to be used as proxies for past deep ocean temperature. This specimen, Bulimina sp., measures about 400 x 250 micrometers (0.02 x 0.01 inch.) (Photo by Joan Bernhard, Woods Hole Oceanographic Institution)
- Small culture chambers called "microcosms" hold deep-sea benthic foraminifera in carefully controlled physical and chemical conditions. When the cells grow and form new shell material, WHOI scientists Berhnard and McCorkle will link shell composition with the precise growth conditions, "calibrating" the shell thermometer. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)