Beneath Arctic Ice, Life Blooms Spectacularly
Pools of water atop ice act as lenses to focus sunlight
Scientists have discovered a massive bloom of phytoplankton beneath ice-covered Arctic waters. Until now, sea ice was thought to block sunlight and limit the growth of microscopic marine plants living under the ice.
The amount of phytoplankton growing in this under-ice bloom was four times greater than the amount found in neighboring ice-free waters. The bloom extended laterally more than 100 kilometers (62 miles) underneath the ice pack, where ocean and ice physics combined to create a phenomenon that scientists had never seen before.
Reporting in the June 8, 2012 edition of the journal Science, a multi-institutional team of scientists concluded that ice melting in summer forms pools of water that act like transient skylights and magnifying lenses. These pools focused sunlight through the ice and into waters above the continental shelf north of Alaska, where currents steer nutrient-rich deep waters up toward the surface. Phytoplankton under the ice were primed to take advantage of this narrow window of light and nutrients.
“Way more production is happening under the ice than we previously thought, in a manner that’s very different than we expected,” said Sam Laney, a biologist from Woods Hole Oceanographic Institution (WHOI). Just as a rainstorm in the desert can cause the landscape to explode with wildflowers, this research shows that events like pooling melt water can happen on very short timescales in the Arctic yet have major effects on the ecosystem. “If you don’t catch these ephemeral events, you’re missing a big part of the picture.”
Laney was part of the team that made the unexpected discovery on a 2011 expedition led by chief scientist Kevin Arrigo of Stanford University aboard the U.S. Coast Guard icebreaker Healy. It was part of the NASA-funded ICESCAPE program to investigate the impact of climate change in the polar Chukchi and Beaufort seas.
The scientists found themselves in the right place at the right time in early July of 2011, as the Healy made its way above the Arctic Circle and into year-old ice. Atop the meter-thick ice, pools of sky-blue melt water were accumulating as the Arctic summer progressed.
It was Laney’s second summer aboard the Healy studying microscopic plankton in this cold, ice-covered region. MIT/WHOI Joint Program graduate student, Emily Brownlee, was also aboard on her first oceanographic research cruise. From her work station deep within the ship, she was being introduced to the scary grinding sounds of hull breaking ice. The scientific party expected that, as in years past, the waters beneath the ice would have minimal amounts of chlorophyll, the fluorescing hallmark of photosynthetic marine plants. Instead, they observed the opposite: as the ship broke further into the ice, chlorophyll in the dark waters below shot up to levels rarely observed even in the most productive ocean regions. It became evident that there was a phytoplankton bloom of astonishing magnitude happening under the ice.
Optical measurements showed that four times as much light penetrated ice covered by meltwater ponds than ice covered by snow. “Although the under-ice light field was less intense than in ice-free waters, it was sufficient to support the blooms of under-ice phytoplankton, which grew twice as fast at low light as their open ocean counterparts,” the scientists wrote in Science.
The ship conducted transects into the ice pack to determine how far and deep the bloom extended. Measurements of biomass showed the largest part of the bloom occurred far away from the open ocean, under thick ice and close to water upwelling at the continental shelf break, where the shallow coastal shelf plunges steeply into deeper water.
Another member of the ICESCAPE team, WHOI physical oceanographer Bob Pickart, showed that easterly winds churned out by monster storm systems along the Aleutian Islands can reverse the current along the shelf break. The change in circulation drives cold, nutrient-rich water up from the abyss and refreshes the supply of nutrients available to phytoplankton growing near the surface.
“Without a doubt the bloom was enhanced at the shelf break,” he said. As the melt pools introduced light through the ice, phytoplankton in shelf break waters likely experienced something akin to an all-you-can-eat buffet.
What types of organisms participated in the bloom? Laney caught them on camera with an instrument he had brought to sea, the Imaging FlowCytobot, an automated microscopic imaging system invented at WHOI and built by Laney and Imaging FlowCytobot co-developer Rob Olson. (See slideshow.)
Since their return, Laney and MIT/WHOI Joint Program graduate student Emily Brownlee have been working with the Imaging FlowCytobot’s other co-developer and ICESCAPE team member, WHOI biologist Heidi Sosik, to sort through and classify organisms in the millions of images collected by the instrument and unravel intricacies of the under-ice bloom. The images showed that the bloom was not caused by fallout from algae growing on the ice, but rather it contained different organisms that seized the confluence of currents, light, and nutrients.
The Imaging FlowCytobot offers tantalizing insights into the microbial dynamics and food web of this dramatic Arctic algal boom. For example, the images consistently showed some phytoplankton in the bloom in a half-munched state, offering clues to the planktonic grazers that might be eating them.
Discovering the under-ice bloom “was very serendipitous,” Pickart said, but the difficulty of exploring the harsh, remote region also leaves the Arctic wide open for surprises.
Amid the fascinating new questions emerging from images, Brownlee has already reached a solid conclusion from the whole experience: “It made me realize that I really want to keep going to sea.”
Slideshow
Slideshow
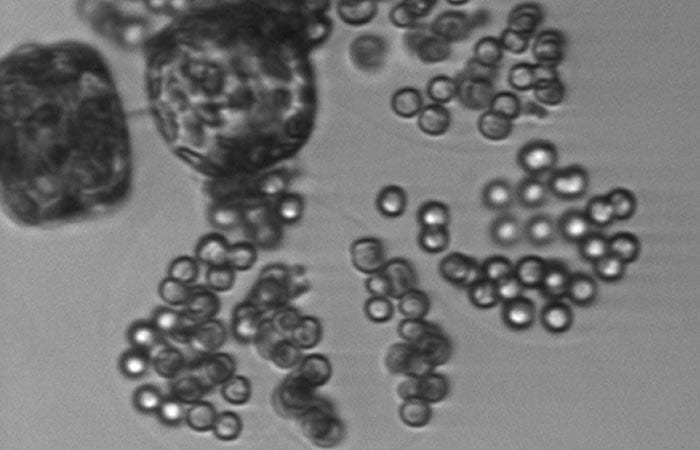
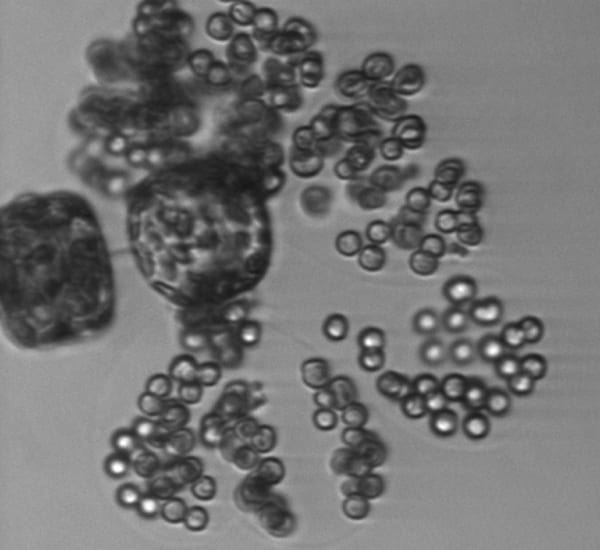
 Tiny round cells of the alga Phaeocystis are only a few micrometers in diameter, but they form large colonies of hundreds of individual cells held together with a gel-like substance. Here, in a rare view, are single cells, doublets, and the beginnings of colony formation. Colony-forming cells are getting a start by attaching to two cells of a larger algae species, Thalassiosira.
Tiny round cells of the alga Phaeocystis are only a few micrometers in diameter, but they form large colonies of hundreds of individual cells held together with a gel-like substance. Here, in a rare view, are single cells, doublets, and the beginnings of colony formation. Colony-forming cells are getting a start by attaching to two cells of a larger algae species, Thalassiosira.
Probably the chain-forming diatom Thalassiosira. Cylindrical cells (seen here on edge) are linked by a central spine that looks like a very faint line. Other long spines protrude from the cells.
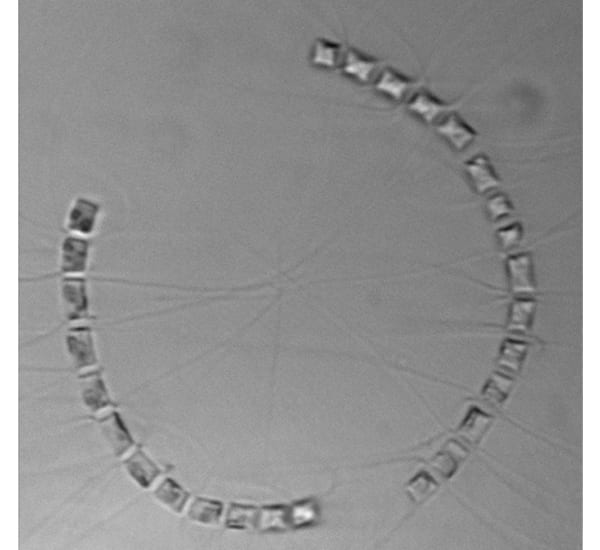
Two chains of the diatom Chaetoceros socialis curve toward each other. Long spines come from each cell's four corners and intertwine to attach it to its neighbor cells' spines. One spine at each end of a cell is longer than the other, and these intertwine with the long spines of other cells, forming three-dimensional ball-like clusters of chains—hence the name 'socialis', for this 'social' behavior.
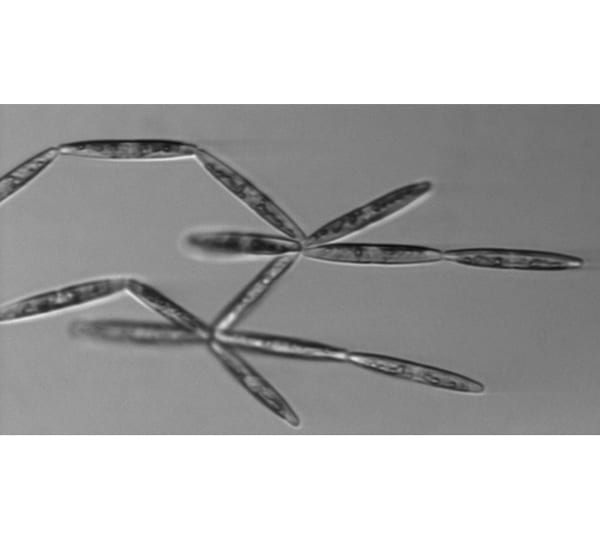
Not all floating algae cells are round. This is a pennate (wing-shaped) diatom called Pseudonitzschia, each cell about 70 micrometers long. They form chains, but the chains aren't necessarily linear. They were found in brine channels inside the sea ice.
 Another pennate diatom, Pleurosigma, tapers elegantly at either end. Small clumps of material inside the cell are the chlorophyll-containing chloroplasts, the organelles where photosynthesis takes place.
Another pennate diatom, Pleurosigma, tapers elegantly at either end. Small clumps of material inside the cell are the chlorophyll-containing chloroplasts, the organelles where photosynthesis takes place.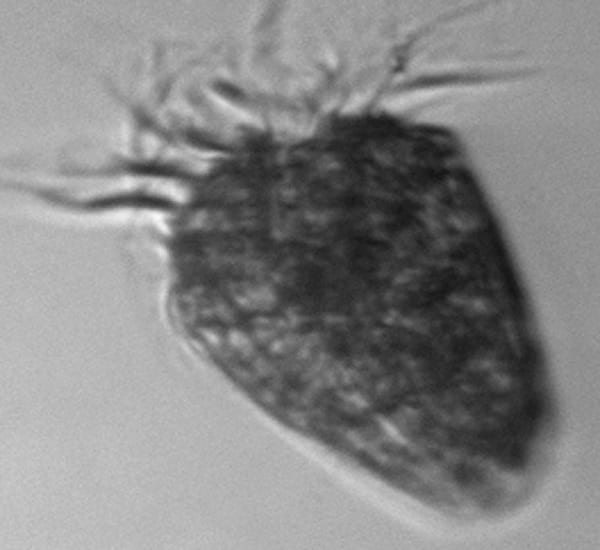
This strawberry-shaped cell, bearing a crown of hair-like cilia, is not part of the phytoplankton, but instead a single-celled predator on other cells in the bloom. It is a ciliate, part of the group sometimes called protozoa.
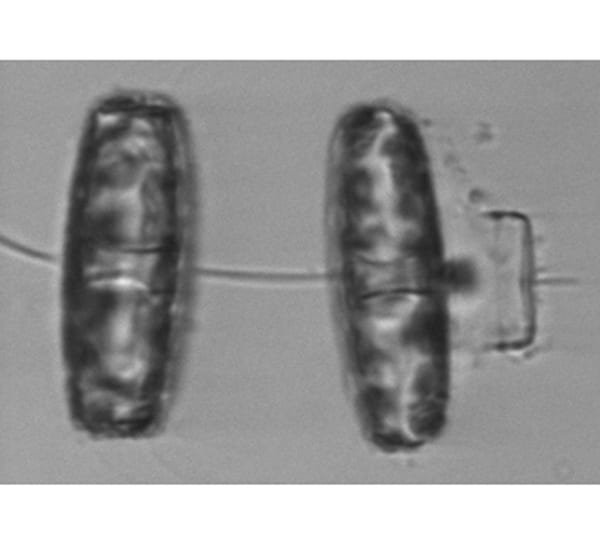
Diatom cells have hard inner and outer covers, like larger and smaller lids. They reproduce by both asexual cell division and a sexual form of reproduction. The Thalassiosira cell on the right has undergone sexual reproduction, leaving behind the "lid" of the parent cell—visible as the thin bracket-shaped object on the far right.

Two pairs of Thalassiosira diatoms 'caught in the act' of asexual reproduction. The pair on the left has just finished binary cell division, their usual, asexual form of reproduction. The thin gap between these two daughter cells indicates that division is complete and these are two independent individuals. The pair on the right is still dividing—still building, WHOI biologist Sam Laney says, the new cell walls and "exterior siding" that will separate them.
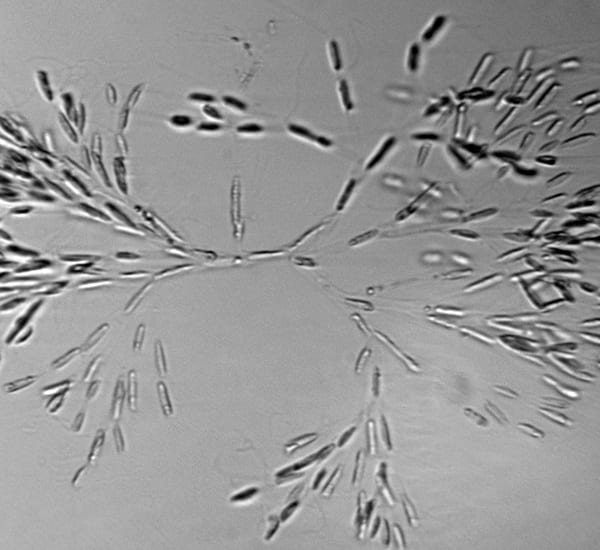 Something different—a delicate fan-shaped colony of a golden-colored algae, called Dinobryon. The three-dimensional structure means that some cells are in focus, while others are not.
Something different—a delicate fan-shaped colony of a golden-colored algae, called Dinobryon. The three-dimensional structure means that some cells are in focus, while others are not.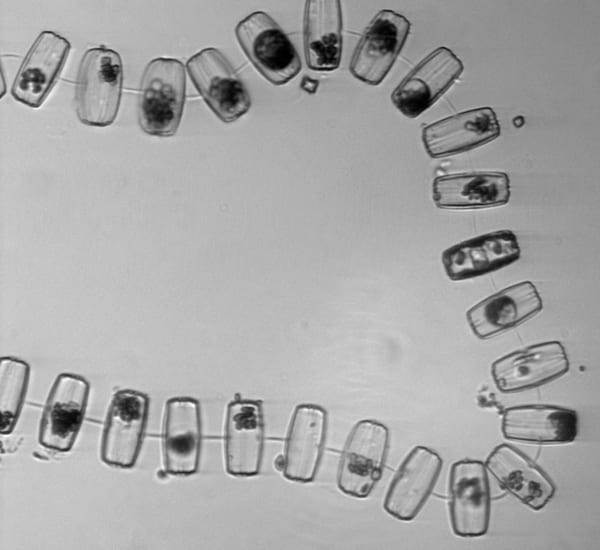
This diatom chain, says Laney, is not healthy. The cells are more or less empty shells, not packed with cytoplasm as in the earlier Thalassiosira images. "The shriveled cells here are a clear indicator that environmental conditions have taken a turn for the worse," he said, "presumably at the end of the bloom when the nutrients are all used up."
 U.S. Coast Guard rescue swimmers and polar bear sentinels from the Coast Guard icebreaker Healy stand by as scientists take samples of sea ice and underlying seawater properties during NASA's 2011 ICESCAPE cruise in the Chukchi and Beaufort Seas. To their surprise, scientists discovered a bloom of phytoplankton in presumably dark regions beneath the ice. The scientists concluded that the tourquoise pools of water atop melting ice in summer act like transient skylights and magnifying lenses focusing sunlight into water beneath the ice. (Sam Laney, Woods Hole Oceanographic Institution)
U.S. Coast Guard rescue swimmers and polar bear sentinels from the Coast Guard icebreaker Healy stand by as scientists take samples of sea ice and underlying seawater properties during NASA's 2011 ICESCAPE cruise in the Chukchi and Beaufort Seas. To their surprise, scientists discovered a bloom of phytoplankton in presumably dark regions beneath the ice. The scientists concluded that the tourquoise pools of water atop melting ice in summer act like transient skylights and magnifying lenses focusing sunlight into water beneath the ice. (Sam Laney, Woods Hole Oceanographic Institution)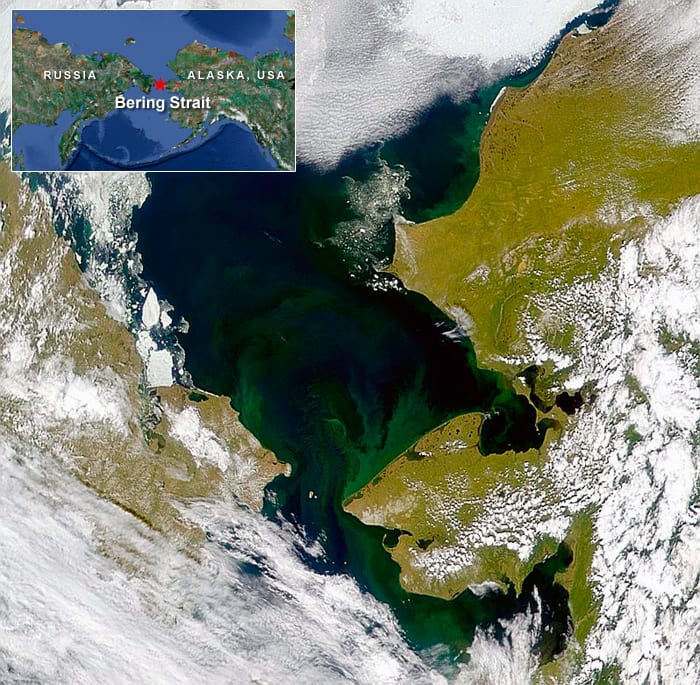 A rare cloud-free satellite image of the Chukchi Sea, just north of the Bering Strait shows coastal areas where phytoplankton bloom (green) when sea ice melts in summer. Climate models project that changes in the ice cover may accelerate in the future Scientists in the NASA-funded ICESCAPE project made two expeditions to the seas in the summers of 2010 and 2011 to explore potential impacts of climate change on the region's ecosystem. The ICESCAPE cruises sampled most of the different biogeochemical and optical regimes in this region, to understand better how biological and optical variability in this complex coastal region affects the distributions of ocean color properties that can be measured from sensors on spacecraft. (NASA)
A rare cloud-free satellite image of the Chukchi Sea, just north of the Bering Strait shows coastal areas where phytoplankton bloom (green) when sea ice melts in summer. Climate models project that changes in the ice cover may accelerate in the future Scientists in the NASA-funded ICESCAPE project made two expeditions to the seas in the summers of 2010 and 2011 to explore potential impacts of climate change on the region's ecosystem. The ICESCAPE cruises sampled most of the different biogeochemical and optical regimes in this region, to understand better how biological and optical variability in this complex coastal region affects the distributions of ocean color properties that can be measured from sensors on spacecraft. (NASA) WHOI Biologist Sam Laney and research technician Emily Peacock on "ice liberty" during the 2010 ICESCAPE cruise. For morale, the captain of the Healy allowed one recreational afternoon during the cruise for scientists and crew to step off the ship and enjoy walking around on the polar icecap. Here, Laney and Peacock are literally standing on the Arctic Ocean, with more than 1,000 meters of water underneath them. (Photo by Karen Romano Young)
WHOI Biologist Sam Laney and research technician Emily Peacock on "ice liberty" during the 2010 ICESCAPE cruise. For morale, the captain of the Healy allowed one recreational afternoon during the cruise for scientists and crew to step off the ship and enjoy walking around on the polar icecap. Here, Laney and Peacock are literally standing on the Arctic Ocean, with more than 1,000 meters of water underneath them. (Photo by Karen Romano Young)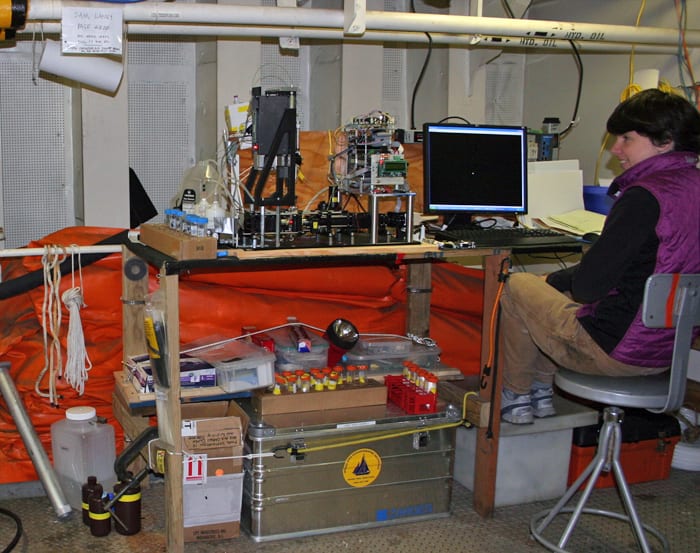 WHOI technician Emily Peacock operates the seagoing Imaging FlowCytobot, an underwater microscope developed by WHOI scientists that took images of microscopic plant and animal life in polar waters (see slideshow below). The instrument collects a wealth of data on what species are living where and when. To get seawater samples of phytoplankton directly out of the ocean (without having them first pass through the high-volume pump that supplies the ship's internal science seawater supply) the FlowCytobot needed to be placed in a compartment deep within the ship, near a port in the hull where a supplementary seawater intake could be placed. The only available compartment for this was the "Hose Reel Room" at the very aft end of the ship, directly above the propellers where refueling hoses are stored. When breaking ice, the Healy's twin propellers would send meter-sized chunks directly into the hull plating immediately below this compartment. Emily here enjoys a quiet moment but ear protection and warm coats were de rigeur when working in this compartment during icebreaking operations. (Photo by Karen Romano Young)
WHOI technician Emily Peacock operates the seagoing Imaging FlowCytobot, an underwater microscope developed by WHOI scientists that took images of microscopic plant and animal life in polar waters (see slideshow below). The instrument collects a wealth of data on what species are living where and when. To get seawater samples of phytoplankton directly out of the ocean (without having them first pass through the high-volume pump that supplies the ship's internal science seawater supply) the FlowCytobot needed to be placed in a compartment deep within the ship, near a port in the hull where a supplementary seawater intake could be placed. The only available compartment for this was the "Hose Reel Room" at the very aft end of the ship, directly above the propellers where refueling hoses are stored. When breaking ice, the Healy's twin propellers would send meter-sized chunks directly into the hull plating immediately below this compartment. Emily here enjoys a quiet moment but ear protection and warm coats were de rigeur when working in this compartment during icebreaking operations. (Photo by Karen Romano Young) The Coast Guard icebreaker Healy took scientists to explore ecosystems of the Chucki and Bering Seas in 2010 and 2011. Healy is nominally a "cutter" in Coast Guard parlance and its main mission is polar science support, although Healy routinely responds to emergency situations in the Bering and Chukchi where it is sometimes the only Coast Guard cutter for hundreds of miles. Healy is the largest ship in the US Coast Guard's "red hull fleet" (large icebreakers) and it dwarfs all other cutters in the Coast Guard's more numerous "white hull fleet." Healy can accommodate up to 50 scientists in addition to its crew of approximately 90, for science missions lasting more than two months. (Photo by Karen Romano Young)
The Coast Guard icebreaker Healy took scientists to explore ecosystems of the Chucki and Bering Seas in 2010 and 2011. Healy is nominally a "cutter" in Coast Guard parlance and its main mission is polar science support, although Healy routinely responds to emergency situations in the Bering and Chukchi where it is sometimes the only Coast Guard cutter for hundreds of miles. Healy is the largest ship in the US Coast Guard's "red hull fleet" (large icebreakers) and it dwarfs all other cutters in the Coast Guard's more numerous "white hull fleet." Healy can accommodate up to 50 scientists in addition to its crew of approximately 90, for science missions lasting more than two months. (Photo by Karen Romano Young)
Related Articles
Featured Researchers
See Also
- Sam Laney Lab
- Robert Pickart Lab
- Heidi Sosik
- Building an Underwater Microscope An article on Flow Cytobot from Oceanus magazine
- ICESCAPE Mission
