Tracking an Elusive Chemical: Estrogens
What impacts might these hormones have in the coastal ocean?
On a crisp October morning, our small boat bobbed gently 10 miles offshore. The sun glinted off the dark blue surface of Massachusetts Bay and directly below us, all of Boston’s sewage was surging into the ocean. Back on shore the city was waking up and flushing the toilet, and the ensuing “morning rush” had just begun its 19-hour journey to this place. Out of sight, this plume of sewage effluent would be easy to ignore if it didn’t contain hard-to-detect chemicals that could have impacts on our health and the environment.
We had spent weeks preparing for our first sampling trip, and I crossed my fingers as we began setting up the equipment we would use to stalk an elusive chemical in the ocean.
If you are a human or any other animal with a backbone, your body makes estrogen. And every day, you send some portion down the toilet to your local sewage treatment plant or septic system. Treated or not, these waste streams ultimately flow into nearby groundwater, rivers, lakes, and coastal waters that often supply the water we drink and the fish we eat.
Estrogens are a family of hormones that are essential for growth and development, and, notably, for determining whether you are a female or a male. Exposure to even small amounts of “extra” estrogen can have profoundly negative health effects, giving rise to male fish with female sex organs, for example.
In 2001, to test just how potent estrogens could be, researchers at Fisheries and Oceans Canada and the United States Environmental Protection Agency began an experiment. They added a synthetic estrogen commonly found in birth control pills to a pristine lake in Ontario at levels typical of those detected in treated sewage. They found that within two years, the lake’s entire population of fathead minnows had collapsed because they were unable to reproduce.
My own research aims to characterize the amounts, variety, and ultimate fate of estrogens discharged with sewage into Massachusetts Bay. Until now, scientists have primarily focused on a single form of estrogen. However, we are looking for a wide array, including chemical forms that have escaped notice in the past, and we are looking for them at very low concentrations. Fortunately, recent advances in detector technology have given us the ability to pick out estrogens from the muddle of millions of other compounds in seawater.
Is dilution the solution?
On an average day, greater Boston releases 360 million gallons of treated sewage into Massachusetts Bay through a 24-foot-wide pipe that could fit two semi-trailers side by side. This is a tiny fraction of the flow of treated sewage worldwide, but it is huge nonetheless—roughly equivalent to driving seven semis full of treated sewage into the bay every minute of every day.
Below our boat that October day, perhaps only a couple teaspoons worth of estrogens flowed into the water. Massachusetts Bay contains enough water to fill 50 million Olympic-size swimming pools and is continually flushed by water from the neighboring Atlantic Ocean. Mix those few teaspoons of estrogen into the bay and you have one extremely dilute solution of estrogen. But a dilute solution is well worth studying because estrogens are potent at minuscule (parts per trillion) concentrations. We still know very little about the kinds and amounts of estrogens that end up in our groundwater, rivers, and lakes. We know even less about estrogens in seawater.
For many years, Boston’s treated sewage was released directly into its harbor, and contaminants and excessive nutrients in the sewage visibly impaired the health of this relatively small body of water. In 2000, after several years of research, planning, and construction, and at great expense, Boston’s treated sewage was rerouted offshore through 9.5 miles of bedrock and a long series of 55 “diffusers” that empty onto the floor of Massachusetts Bay, under 100 feet of water. Here, the treated effluent is mixed and diluted into a much larger body of water.
If dilution were the only process at work, then determining estrogen concentrations would be straightforward. Concentrations would depend on the total amount of estrogens discharged with sewage, the volume of Massachusetts Bay, and the average time the water spends there. But some estrogens are removed from the water over time by a variety of natural processes. Estrogens can get swept away by currents. They may also stick to particles and sink to the ocean floor, accumulate in animal tissues, be altered by bacteria, or be destroyed by sunlight in surface waters.
And it is likely that treated sewage isn’t the only source of estrogen. When it rains, pulses of estrogens probably enter the coastal ocean as an overflow of storm water temporarily overwhelms sewage treatment plants, and they are forced to shunt untreated or partially treated sewage into the harbor.
Which of these processes are most important? To find out, we first have to measure how much of each type of estrogen is in the bay.
A wide variety of estrogens
There is a maxim in the field of toxicology that says, “The dose makes the poison.” This is why eating the trace amounts of cyanide in apple seeds is safe, but drinking too much water can kill you. The saying holds for the concentration of estrogen in coastal waters, but distinguishing “harmful” from “safe” is a real challenge—particularly when that threshold lies near the lower limit of detection. This is a common problem for potent substances such as estrogens.
In my current research, with advisors Philip Gschwend at the Massachusetts Institute of Technology and Timothy Eglinton at the Swiss Federal Institute of Technology in Zurich, we are developing methods to detect and identify estrogens. First we pump 10 to 20 gallons of seawater through thin porous disks about the size and thickness of a CD, made from a material that is “sticky” to estrogens and many other compounds in the water. We then return to the lab and soak our disks in a small amount of alcohol, which pulls estrogens off the disks and into the liquid. By evaporating most of the alcohol, we can concentrate our sample down to 1 milliliter (less than a ¼ teaspoon) before injecting 1 percent of that solution into an instrument called a liquid chromatograph mass spectrometer. The LC-MS, as it is referred to, can separate and detect different estrogens based on their specific structures and masses.
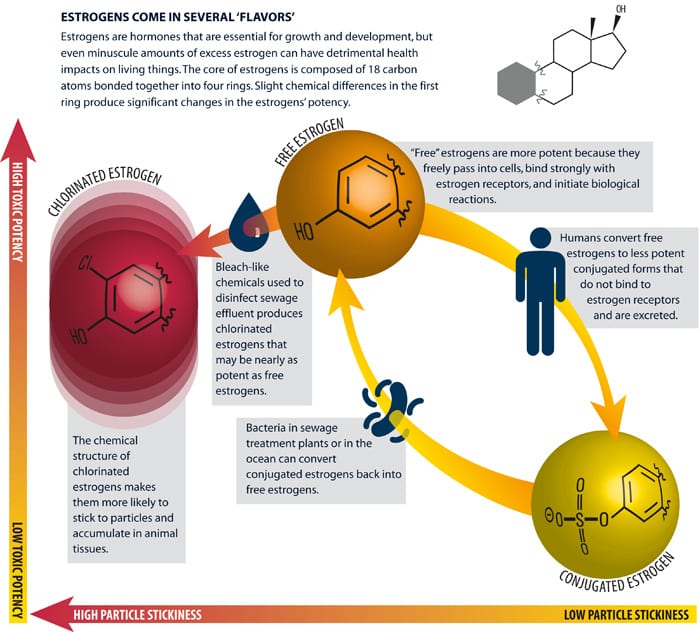
Estrogens take a variety of forms, the most potent of which are “free” estrogens—so called because they freely pass into cells and bind with estrogen receptors, initiating a cascade of biological responses. One particularly potent synthetic free estrogen, 17α-ethynylestradiol, has been widely used in birth control pills since the 1960s. Hans Inhoffen and his colleagues at Schering A.G. in Berlin first synthesized 17α-ethynylestradiol in 1938 by adding two extra carbon atoms to a standard form of estrogen. This slight difference in chemical structure makes 17α-ethynylestradiol more potent than natural estrogens, and more likely to persist in the environment.
Our bodies regularly produce a fresh supply of estrogens. To maintain a balance, certain enzymes convert our existing free estrogens into “conjugated” forms that can be excreted in feces and urine. (The conversion involves attaching a sulfate or glucuronide group to an oxygen atom of the free form.) The resulting conjugated estrogen is no longer of the proper size or chemical character to bind to an estrogen receptor, which is why these forms are biologically inactive. That seems like good news: We are excreting inactive forms. It turns out, though, that bacteria in sewers and sewage treatment plants can convert conjugates back into potent free estrogens.
Sewage treatment alters estrogens in other ways as well. One of the last stages of treatment is disinfection; it is designed to kill harmful germs before effluent is discharged to the environment. Most treatment plants accomplish this by adding what amounts to bleach to the effluent. There is evidence that this step produces chlorinated estrogens that can be nearly as potent as the free forms. What’s more, the chemical structure of chlorinated estrogens makes them more likely to accumulate in animal tissues.
A long research row to hoe
Knowing the concentration or “dose” of each form of estrogen—and there might be dozens of them—in Massachusetts Bay is a critical step in determining whether these chemicals might be posing a threat to local populations of organisms such as shellfish, flounder, or right whales. In general, if concentrations exceed the “no-harm” threshold set by toxicologists, then it may be desirable to move sewage pipes, alter treatment technologies, or work with pharmaceutical companies to redesign drugs that quickly degrade in treatment plants and receiving waters.
Massachusetts Bay is just one place where understanding the chemical forms and behaviors of estrogens could lead to insights about how to design cost-effective solutions. The work here can also help us make predictions for many of the other large coastal cities such as Los Angeles, Hong Kong, and Sydney, which release sewage into nearby coastal waters.
But first things first. By dusk, our precious first samples are secure in a small Igloo cooler. As we motor back to Boston in the fading light, I am charting the days of analyses ahead of me. I’m eager to see if there’s an estrogen story stuck to the disks in our cooler.
This research is funded by a grant from the Coastal Ocean Institute at WHOI and by the EPA STAR graduate fellowship program.
From the Series
Slideshow
Slideshow
 (Illustration by Amy Caracappa-Qubeck, Woods Hole Oceanographic Institution)
(Illustration by Amy Caracappa-Qubeck, Woods Hole Oceanographic Institution) MIT/WHOI graduate student David Griffith stands next to an actual-size model of a diffuser at the Massachusetts Water Resources Authority Deer Island sewage treatment plant. Treated sewage is piped offshore. Diffusers discharge it near the seafloor in deep water, where it mixes with a large body of water. (Photo courtesy of David Griffith, MIT/WHOI Joint Program)
MIT/WHOI graduate student David Griffith stands next to an actual-size model of a diffuser at the Massachusetts Water Resources Authority Deer Island sewage treatment plant. Treated sewage is piped offshore. Diffusers discharge it near the seafloor in deep water, where it mixes with a large body of water. (Photo courtesy of David Griffith, MIT/WHOI Joint Program) Back in the lab at WHOI, David Griffith analyzes samples of seawater he collected in Massachusetts Bay to detect minute quantities of estrogens in the ocean. (Photo by Ken Kostel, Woods Hole Oceanographic Institution)
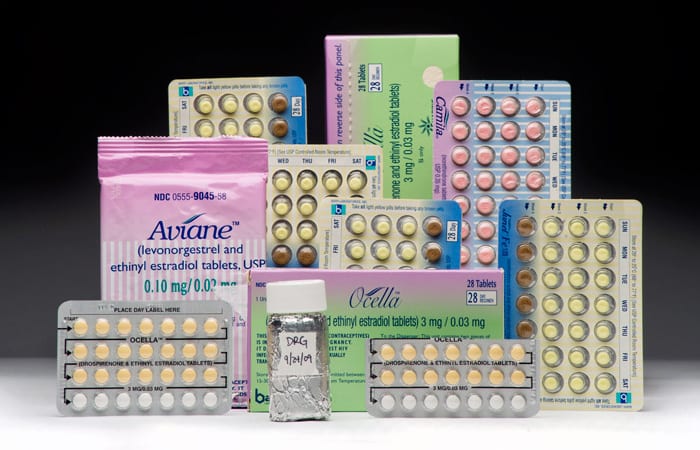
Back in the lab at WHOI, David Griffith analyzes samples of seawater he collected in Massachusetts Bay to detect minute quantities of estrogens in the ocean. (Photo by Ken Kostel, Woods Hole Oceanographic Institution)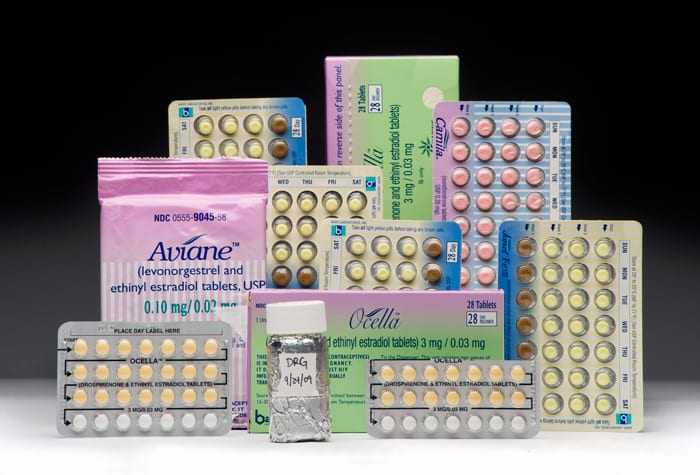 Estrogens are a family of hormones that are essential for growth and development and are produced naturally by living things including humans. Other estrogens are produced synthetically for products such as birth control pills, and these estrogens can eventually enter the ocean when pills are flushed down toilets or thrown away. Synthetic estrogens in birth control pills also enter the ocean after being excreted from the body and flushed down toilets. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
Estrogens are a family of hormones that are essential for growth and development and are produced naturally by living things including humans. Other estrogens are produced synthetically for products such as birth control pills, and these estrogens can eventually enter the ocean when pills are flushed down toilets or thrown away. Synthetic estrogens in birth control pills also enter the ocean after being excreted from the body and flushed down toilets. (Photo by Tom Kleindinst, Woods Hole Oceanographic Institution)
Related Articles
Featured Researchers
See Also
- David Griffith
- Collapse of a fish population after exposure to a synthetic estrogen By K. Kidd, et al, Proceedings of the National Academy of Sciences, May 2007
- Boston Harbor and Massachusetts Bay from the Massachusetts Water Resources Authority
- How the Sewer System Works from the Massachusetts Water Resources Authority
- Philip M. Gschwend Ford Professor of Civil and Environmental Engineering at MIT
- Timothy Eglinton Professor, ETH Zurich
