Deep-sea Tubeworms Get Versatile ‘Inside’ Help
Scientists find first known organism that makes organic carbon by two different means

Estimated reading time: 6 minutes
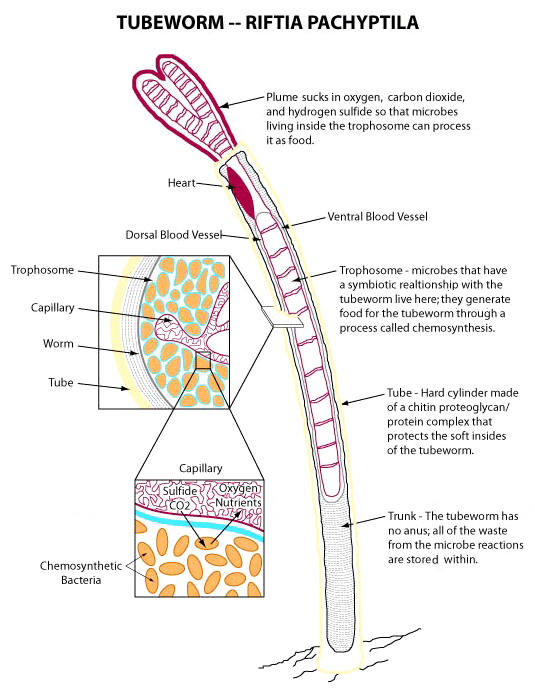
Cross sections of the tubeworm Riftia pachyptila. (Courtesy of Enduring Resources for Earth Science Education)
When scientists found lush thickets of 6-foot-tall, red-tipped tubeworms on the seafloor in 1977, they realized that life could thrive without sunlight in extreme environments.
When they discovered that the tubeworms had no mouth, digestive tract, or anus, they learned that bacteria live inside the tubeworms’ bodies in a remarkable organ called a trophosome. In exchange for a fertile place to live, the bacteria convert carbon dioxide into organic carbon by using chemical energy—much the way chloroplasts provide nutrition for plants via photosynthesis using the sun’s energy.
Now, a team of 12 scientists has found that the symbiotic bacteria on which the gutless, buttless tubeworms depend are surprisingly versatile: They can use two different ways to metabolize carbon dioxide and can switch back and forth to accommodate fast-changing environmental conditions. The findings were reported in the Jan. 12 issue of the journal Science.
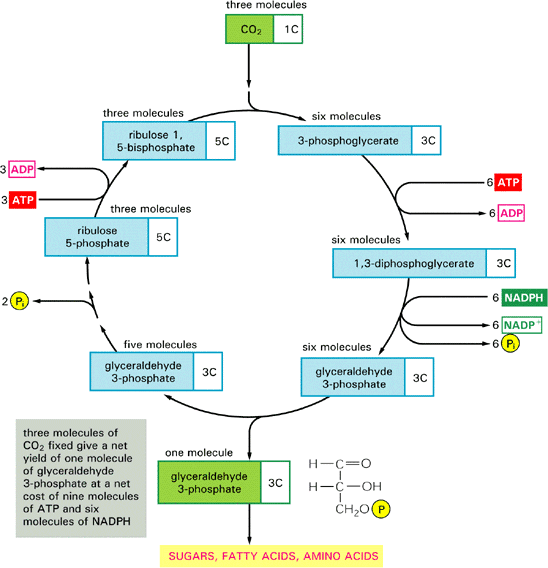
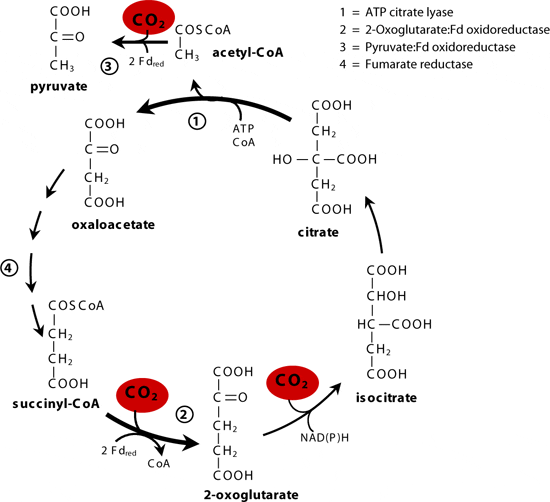
Scientists have known microorganisms that use the so-called Calvin cycle to convert, or “fix,” carbon dioxide into a form that livings things can use, and other microorganisms that use another metabolic pathway known as the reductive tricarboxyclic (rTCA) cycle. “But this is the first occurrence of an organism that can switch between the two carbon fixation pathways, or even use both at the same time,” said Stefan Sievert, a microbiologist at Woods Hole Oceanographic Institution (WHOI) and co-author of the study led by Thomas Schweder and Stephanie Markert of the Institute of Marine Biotechnology in Germany. Co-authors included scientists from the Max-Planck-Institute for Infection Biology and Ernst-Moritz-Arndt University in Germany and Scripps Institution of Oceanography, University of California Santa Cruz, and SymBio Corp. in California.
When scientists found lush thickets of 6-foot-tall, red-tipped tubeworms on the seafloor in 1977, they realized that life could thrive without sunlight in extreme environments.
When they discovered that the tubeworms had no mouth, digestive tract, or anus, they learned that bacteria live inside the tubeworms’ bodies in a remarkable organ called a trophosome. In exchange for a fertile place to live, the bacteria convert carbon dioxide into organic carbon by using chemical energy—much the way chloroplasts provide nutrition for plants via photosynthesis using the sun’s energy.
Now, a team of 12 scientists has found that the symbiotic bacteria on which the gutless, buttless tubeworms depend are surprisingly versatile: They can use two different ways to metabolize carbon dioxide and can switch back and forth to accommodate fast-changing environmental conditions. The findings were reported in the Jan. 12 issue of the journal Science.
Scientists have known microorganisms that use the so-called Calvin cycle to convert, or “fix,” carbon dioxide into a form that livings things can use, and other microorganisms that use another metabolic pathway known as the reductive tricarboxyclic (rTCA) cycle. “But this is the first occurrence of an organism that can switch between the two carbon fixation pathways, or even use both at the same time,” said Stefan Sievert, a microbiologist at Woods Hole Oceanographic Institution (WHOI) and co-author of the study led by Thomas Schweder and Stephanie Markert of the Institute of Marine Biotechnology in Germany. Co-authors included scientists from the Max-Planck-Institute for Infection Biology and Ernst-Moritz-Arndt University in Germany and Scripps Institution of Oceanography, University of California Santa Cruz, and SymBio Corp. in California.
Solving a longstanding conundrum
Bacteria like these probably played fundamental roles in the evolution of life on Earth. But their environment—both inside living tubeworms and around deep-sea hydrothermal vents that spew hot, metal-rich fluids—is difficult to imitate in laboratories, so scientists have never been able to culture them.
The team used a novel approach: They analyzed the bacteria’s genome along with their proteome, the proteins transcribed and produced by the genes. (The genome is like the bacteria's blueprint, while the proteome is, in a way, the bacteria’s tool box, Sievert said.) The proteome revealed telltale enzymes, which the bacteria use to harness chemical energy and to fix inorganic carbon.
The combined genomic and proteomic approaches offer a valuable way to investigate the metabolic capabilities and history of these microorganisms, said Charles Fisher of Pennsylvania State University and Peter Girguis of Harvard University, who wrote a perspective article on the research in Science.
The new study solves one mystery that had been puzzling scientists for decades. They had found that tubeworm tissues contain more of a heavier stable carbon isotope than expected if the Calvin cycle were the only one at work. Use of the rTCA cycle explains this conundrum, because it results in the incorporation of more of the heavy stable carbon isotope, compared to the Calvin cycle, Sievert said.
“We were always suspicious that something else was going on and that maybe the Calvin cycle wasn’t the whole story,” said Sievert, who, together with then-WHOI postdoctoral scholar Michael Hügler (now at University of Kiel, Germany), provided essential data to prove that the rTCA cycle operated in the symbionts.
The Calvin cycle works with plenty of oxygen around, Sievert explained, but requires substantially more energy than the rTCA cycle, which, on the other hand, is inhibited by higher oxygen concentrations. Such metabolic flexibility is an asset in habitats dependent on the fluctuating flows of fluids emanating from hydrothermal vents, he said.
A relationship with give and take
For a long time, the means by which the tubeworms (Riftia pachyptila) acquired the symbionts had remained a mystery as well, with many investigators thinking that the worms may pick up the bacteria in their larval stage, when the worms still have a mouth. However, just last year, Fisher and Andrea Nussbaumer and Monika Bright of the University of Vienna discovered that the bacteria enter through the tubeworms’ skin in a process that is akin to an infection.
Once the bacteria get inside the tubeworms, both benefit from this unusual arrangement, which is called endosymbiosis (from “endo,” meaning “inside”). The tubeworms’ feather-like red plumes act as gills, absorbing oxygen from seawater and hydrogen sulfide from vent fluids. This feat is accomplished by a special type of hemoglobin in their blood that can transport oxygen and sulfide at the same time (human hemoglobin transports only oxygen). The bacteria inside the tubeworms oxidize hydrogen sulfide to create energy. The tubeworms get a steady supply of organic carbon and can grow prolifically, tacking on roughly 31 inches (80 centimeters) of white tube to their bodies every year.
The mysteries of tubeworms and their endosymbiotic microbes only continue to grow, Sievert said. In certain situations, the endosymbionts may even burn internally stored carbon, Sievert said, giving the bacteria and the tubeworms even more metabolic flexibility to adapt to fluctuating conditions.
“How all this is regulated is currently not well understood,” Sievert said. "Clearly, this study has opened a new door, paving the way for exciting discoveries to come.”
The research was funded by Deutsche Forschungsgemeinschaft, the U.S. National Science Foundation, the U.S. National Aeronautics and Space Administration's Astrobiology Institute, the Woods Hole Oceanographic Institution, and the University of California.