Another Greenhouse Gas to Watch: Nitrous Oxide
Where are steadily rising levels of the gas coming from?
There’s a greenhouse gas whose concentration is on the rise because of human activities. But it’s not the one you’d expect: it’s nitrous oxide (N2O), also known as laughing gas. It’s been accumulating in the atmosphere since the 1700s, and it’s powerful and persistent. One molecule of N2O has the same greenhouse warming power of 300 molecules of carbon dioxide. Once that N2O molecule gets into the upper atmosphere, it can stay there for more than 100 years before it’s destroyed naturally.
Fortunately, air has about 1,000 times less N2O than carbon dioxide. But the rise in N2O has accelerated over the past two decades. And while we know where the excess carbon dioxide is coming from, we don’t know precisely how N2O is produced. That’s information we’ll need to know in order to curb future N2O production.
“As the U.S. gets serious about controlling greenhouse gas emissions, we need to figure out how and where N2O is made,” said Kevin Kroeger, a scientist at the U.S. Geological Survey (USGS) in Woods Hole.
Every year an estimated 17 million tons of nitrogen are released to the atmosphere as N2O, according to the International Panel on Climate Change. The source of more than half of this N2O is probably soil, where scientists have tended to focus their studies. The rest comes from the ocean—where measurements have been harder to make. But as we take a closer look, we are realizing that N2O production in the ocean is more important than we used to think.
“People are realizing that soil isn’t the end of the N2O story,” said USGS scientist John Crusius. Kroeger, Crusius, and colleagues John Bratton and Eric Sundquist are leading efforts to measure coastal N2O emissions. The USGS team seeks to answer questions such as: Where is the coastal N2O coming from and how much is there?
At the Woods Hole Oceanographic Institution (WHOI), we’re investigating other pieces of the puzzle: Which organisms are making N2O in the ocean? How are they doing it? Are ocean conditions changing to produce more N2O?
In the oceans and in soil, the primary players are microbes, which, as they go about their lives, transform certain nitrogen compounds into N2O. Humans have dumped huge amounts of these compounds into the soil and coastal waters, sending the microbes into N2O overdrive.
Think globally, act coastally
When people add nitrogen-rich fertilizers to farmland and lawns, the nitrogen dissolves in groundwater and runoff that eventually flows into the ocean. (See Tracking Nitrogen’s Elusive Trail Through the Ocean). In heavily settled coastal areas, nitrogen also seeps out of septic tanks (human waste, like other kinds of manure, is rich in nitrogen) or vaporizes over land and rains out or blows out to sea.
Coastal areas represent a small part of the total ocean, but the human impact on them is disproportionately high. Heavily populated coastlines such as Cape Cod get hit with a particularly heavy nitrogen load.
So far, the USGS group has focused on the intertidal zone, the region between low and high tide. A lot of N2O could be coming out of this area, but it has been overlooked because most N2O measurements are either made in soil or in seawater.
“Coastal (N2O) fluxes in general are not that well understood and may be underappreciated,” Crusius said. The USGS team’s preliminary measurements in West Falmouth Harbor on Cape Cod show that in some areas, N2O is released from sediments at a rate that’s 60 times faster than previous reports on other coastal areas.
Compared to the open ocean, the conditions on the coast are less uniform and change quickly. The trick for these scientists will be to make enough measurements across a large enough area and over a long enough time span to be able to say how much N2O is leaking out of the zone annually.
“Although we might see a significant source in one area, we still have to ask ourselves ‘How big is the area in the grand scheme of things?’” says Crusius.
To catch N2O as it’s produced, the USGS team puts airtight chambers over sand exposed at low tide and then measures how quickly the gas builds up. To make N2O measurements directly on coastal air samples, they’re going to use an instrument called a photoacoustic field gas analyzer.
The device exploits N2O’s ability to absorb infrared radiation—the same property that makes it a powerful greenhouse gas. The N2O molecules vibrate faster when they are exposed to infrared energy. The analyzer amplifies the sounds of the vibrating molecules in a gas sample. A louder sound means more N2O.
Unusual suspects
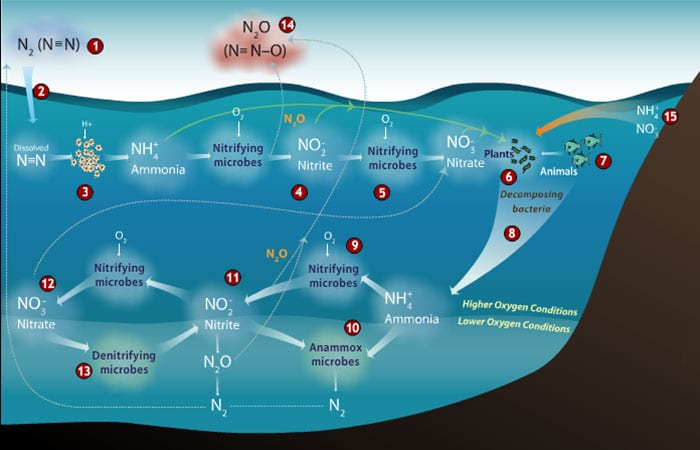
At WHOI, I am taking a closer look at the microbial “personalities” involved in making N2O. It turns out that there are two major groups of microbes that can make N2O. These two groups carry out very different chemical processes, but they depend on each other because they are part of a larger nitrogen cycle that needs them both to continue spinning.
The two groups of N2O-making microbes are called “nitrifiers” and “denitrifiers.” The nitrifiers convert ammonia (NH3) to nitrite (NO2–), a process that gives off a small amount of N2O and gives the microbes the energy to convert carbon dioxide into their cell components (a lot like plants do during photosynthesis).
Nitrifiers can only do this conversion when there is oxygen around. The relationship between oxygen and nitrifier N2O production isn’t straightforward though. For reasons we don’t fully understand yet, when oxygen levels in their vicinity drop, the nitrifiers actually increase their N2O production.
The consequences could be significant. The excess nitrogen that people are adding to coastal waters can stimulate blooms of tiny marine plants (phytoplankton), whose decomposition ultimately depletes the oxygen in the water. The result is a high-nitrogen, low-oxygen environment that encourages nitrifiers to make more N2O.
Unlike the nitrifiers, the other N2O producers, the denitrifiers, have trouble doing their thing when there’s oxygen around. To get energy, they change nitrate (NO3–) to nitrogen gas (N2). N2O is an intermediate produced during that chemical reaction and some of it can leak out of the denitrifiers’ cells and into the environment.
So what is the “sweet spot” combination of conditions that make the nitrifiers and denitrifiers produce the most N2O? To figure this out, we need to know where these two groups of microbes tend to thrive and how each contributes to overall N2O production.
This isn’t easy, because in many areas where N2O is produced, it’s possible for nitrifiers and denitrifiers to coexist. Furthermore, most N2O detection methods can’t distinguish the N2O molecules made by nitrifiers from N2O molecules made by denitrifiers.
I am working with Karen Casciotti, an associate scientist at WHOI to figure out ways to distinguish which N2O molecules come from which microbes. To do this, we’re looking at the isotopes of the nitrogen and oxygen atoms in the N2O molecules. Isotopes are varieties of the same element that have slightly different masses. For N2O, we’re interested in two different isotopic flavors of nitrogen (14N and 15N).
We use a powerful mass spectrometer to separate N2O that contains the heavier nitrogen isotope (15N) from N2O made from the lighter isotope (14N). It turns out that in the asymmetrical N2O molecule (with a structure of N≡N–O), nitrifiers tend to put relatively more 15N into the central nitrogen (N≡15N–O) than denitrifiers do. We can measure that slight preference for 15N when the mass spectrometer blasts the N2O into fragments, giving us a way to tell nitrifier N2O apart from denitrifier N2O.
We started out making these measurements on N2O produced by microbes that we grow in our lab. Now we’re applying the same technique to seawater samples that I collected on a recent cruise in the Atlantic on the research vessel Knorr.
The cruise track went from Brazil to Namibia. It took us through a variety of marine environments, from the low-nutrient subtropical gyre to the coastal waters of southwest Africa, where nutrients upwell and stimulate microbes to produce N2O. The cruise data will help us understand how the transition from low- to high-nutrient conditions influences the rate and mechanisms of N2O production.
Casciotti also has plans to study the controls on coastal N2O production in our own backyard, at the Martha’s Vineyard Coastal Observatory. “It’s ironic that coastal waters are so accessible and yet we know very little about N2O production in most of these areas,” she said.
Although studying N2O is tricky, new methods are making it possible for us to track the elusive gas. That’s good news if we want don’t want this greenhouse gas to have the last laugh.
Caitlin Frame has received support for her research from a National Science Foundation’s Graduate Research Fellowship and a National Defense Science and Engineering Grant. This article was written during a science writing course for graduate students at WHOI, supported by funds from The Henry L. and Grace Doherty Professor of Oceanography.
Related Articles
Featured Researchers
See Also
- Marine Microbial Biogeochemistry at WHOI
- Tracking Nitrogen's Elusive Trail Through the Ocean from Oceanus magazine
- Biogeochemical controls and isotopic signatures of nitrous oxide production by a marine ammonia-oxidizing bacterium By C.H. Frame and K.L. Casciotti in Biogeosciences 2010
- International Nitrogen Initiative



