A Fatal Attraction for Harmful Algae
Clay sticks to algae and sinks, offering a potential solution to an expensive and deadly problem
A Korean scientist once told me a folk tale about an ancient emperor who ordered servants to rid his garden ponds of an algal scum that killed his fish and blemished his kingdom. One perceptive servant noticed that whenever rain washed dirt, sand, and other sediments into the ponds, the water cleared and the fish appeared healthier. As a test, he sprinkled various sediments into the water, discovering that clay was particularly effective. The algae disappeared, and the emperor was happy.
The story offers insight into how people in Asia may have discovered that they could use clay to rid their waters of “red tide” and “brown tide.” Exactly how it works is a more recent discovery.
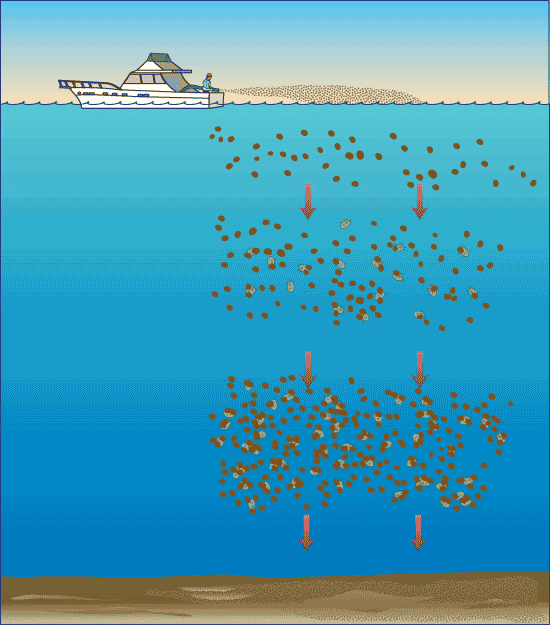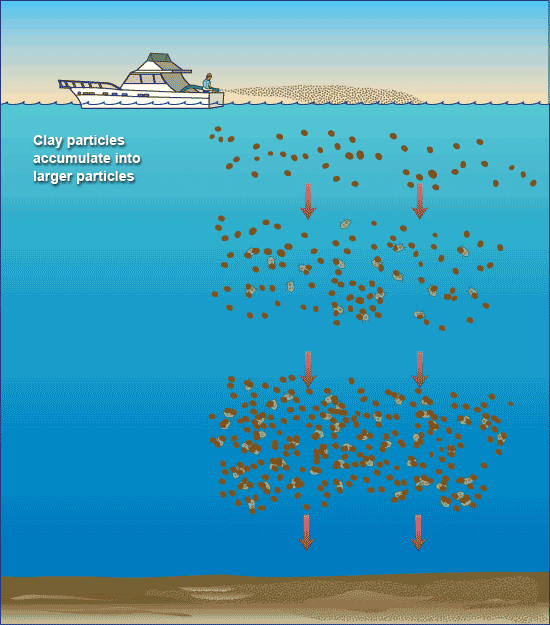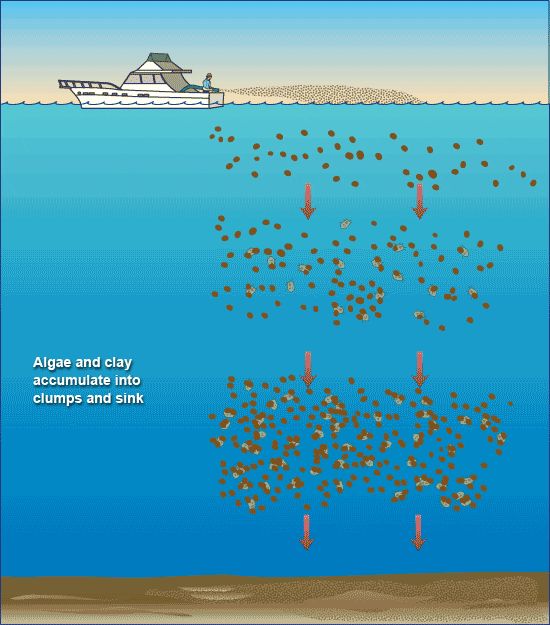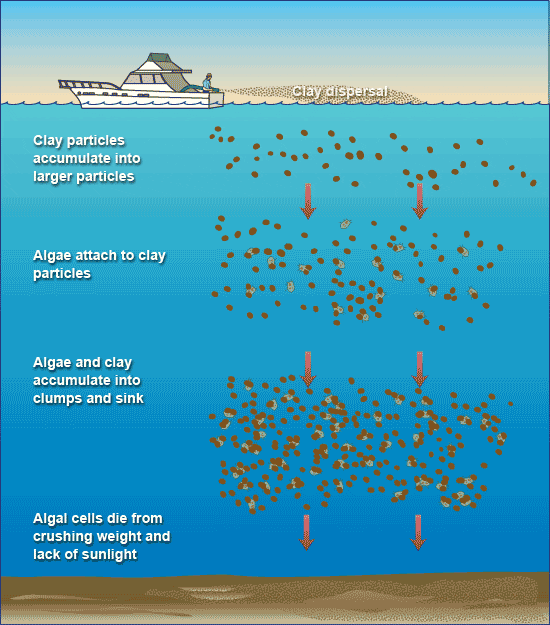
Through laboratory and field experiments, we now know that moistened clay weighs down algal cells, causing them to sink. On the seafloor, the fallen clay crushes many of these microscopic plants. Lack of sunlight kills the rest.
The results of this treatment can be striking. After spraying clay in Japan’s Inland Sea during the late 1980s, researchers noted that blooms of harmful algae quickly declined, water clarity improved, and populations of yellowtail and opaleye fish recovered. After South Koreans began clay treatments in 1996, fishery losses fell from $100 million to $1 million per year.
In the United States, clay treatment is not yet permitted as a control strategy for algal blooms because there are questions about the ecological consequences of this sort of remediation. But as more and more coastal communities struggle with contaminated fish, undermined economies, and public health risks, interest in a solution has soared. Biologists at Woods Hole Oceanographic Institution are part of a team examining clay therapies that could someday be used to mitigate and control harmful algal blooms.
A centuries-old problem
Popularly known as “red tides” or “brown tides” because of how they discolor the water, harmful algal blooms are a natural phenomenon that has been recorded since the book of Exodus. They are caused by species of tiny, plant-like cells—phytoplankton—that produce potent chemical toxins. Fueled by periodic abundances of nutrients in the ocean, these algae proliferate until they cover tens to hundreds of miles of coastal ocean. (See The Growing Problem of Harmful Algae)
These blooms occur worldwide and have particularly affected fisheries in Scandinavia and Asia, reef inhabitants in the Caribbean and South Pacific, and shellfisheries along U.S. coasts. For example, harmful algal blooms caused by the species Karenia brevis along the Gulf Coast of Florida have caused extensive fish kills and contaminated shellfish. In humans, the toxins of this tiny plant can trigger coughing, itchy skin, and respiratory problems.
Given all the trouble linked to these toxic plants, it would seem that people would welcome any technique to stem the tide of algae. But opponents of clay treatment argue that clay threatens bottom-dwelling creatures such as clams and mussels, clogging their filters used for feeding. They also say that clay compromises water quality and too greatly interferes with a naturally occurring process. Proponents counter that the cost is small compared to the devastating impact of algal blooms on fish and the livelihoods of fishermen.
Chemicals, skimmers, and clams
In the 1950s, Florida waters became an early U.S. test bed for remedies to curtail harmful algae. Crop-dusting planes headed offshore, spraying copper sulfate over vast expanses of sea. It proved an expensive and temporary fix, knocking out the algae for just a few days while devastating the fish and shellfish population.
Coastal managers, scientists, and engineers have tried other strategies, with mixed success. Some have tried water filters, pumps, and surface skimmers, only to find that they clogged or suffered mechanical breakdowns. More recently, researchers tested algae-eating clams and mussels—even parasites. There are high-tech proposals to use ultrasonic waves or ozone gas bubbles to cause the algae to burst.
Amidst the innovation, clay appeals as a low-cost, pragmatic solution. It has been the focus of my research for seven years.
A magnetic attraction
Algae stick to clay particles just as a magnet clings to a refrigerator—through the bonding of positive and negative charges on their surfaces. When clay is sprayed onto the surface of waters contaminated with harmful algae, the algal cells attach to the clay, become burdened with its heavy load, and fall to the seafloor. On the way down, the algae-clay particles collide with and gather more algae, forming masses of fluffy debris called “marine snow.”
I started my graduate studies by working with colleagues to identify the best clay for making this algal snow. Not all clay is suited, as some allow algae to pass right through the falling particles without sticking. We tested about 100 types of clay.
My colleagues and I found the most effective clays had a very fine grain and were rich with the mineral montmorillonite. We identified about a dozen pure clays and a few clay-rich sediments that fit this profile. In experiments with Karenia brevis, the toxic algae common off Florida, we found that our clays removed up to 90 percent of the algae. We had similar successes with affected waters from Washington, Texas, and New York.
Impacts on the seafloor
The use of clay may be effective for clearing surface waters, but how does all this falling debris affect the organisms on the bottom? Clams, mussels, and other benthic creatures typically survive on the seafloor by filtering food from overlying water. So before we decide clay is the answer to our algae problems, we need to see how these bottom-dwellers react to clay exposure.
In a series of experiments with colleagues from Canada’s Institute for Marine Biosciences and from Dalhousie University in Nova Scotia, we found that if we sprayed water with small amounts of clay and allowed it to settle, the clams neither died nor differed in their growth compared to untreated clams.
But when we added clay to areas where currents kept clay particles suspended in the water column for longer periods (in our test case, more than two weeks), shell and tissue growth in clams slowed by as much as 90 percent. This suggested that it was important to avoid clay application in places where particles take a long time to settle.
In another experiment at the Environmental Protection Agency’s laboratory in Gulf Breeze, Fla., colleagues compared the harm caused by clay and toxic algae on grass shrimp, sheepshead minnows, and two burrowing crustaceans. In both 4-day and 28-day experiments, clay alone was not lethal. But when combined, the mixture of clay and toxic algae proved deadly.
Since clay treatment may not be more harmful to bottom-dwelling organisms than an untreated bloom, clay could be useful to prevent the impacts of algae near the surface of the water, where many impacts on public health and fish occur.
Taking clay to the real world
In the past few years, we have started testing our results in the field, taking clays and instruments to the sites of a brown-tide event (caused by Aureococcus anophagefferens) near Long Island, N.Y., Karenia brevis red tides in Florida and Texas, and a fish-killing Heterosigma akashiwo outbreak in Washington’s Puget Sound. In most cases, we pumped algae-rich waters from coastal environments into tanks and treated them with clay; sometimes we captured a parcel of water in a natural enclosure where it could then be treated and studied. Each time, we removed more than 70 percent of algae within hours of treatment.
Each success leads us to try experiments on ever-larger scales and under more realistic conditions.
In 2003, we released 130 kilograms (287 pounds) of clay in a cove in Sarasota Bay. We tracked its movement from the surface to the seafloor to see how currents, tides, and other environmental factors would influence the movement, settling rate, and eventual distribution of clay in the sediment.
We found that much of the clay sank within 10 to 30 minutes of dispersal; within an hour, the water column had returned to its pre-treatment clarity. The distribution of clay within our study area appeared to have been determined by the direction and speed of the currents near the bottom, which we measured using current meters and other ocean instruments.
Based on this work, we are confident that we should be able to track a plume in unsheltered waters. Recently we secured permits and permission to try our techniques in the open waters of Sarasota Bay. We are curious to see what happens in a less controlled environment, where the clay and algae will be stirred by the ocean’s natural ebbs, flows, currents, and turbulence.
People accustomed to scouring clay from baseball uniforms, tennis whites, or pottery scrubs are a little surprised when I tell them I am using clay to clear toxic algae from coastal waters. Then I remind them how we already use clay as a cleaning tool. A blob of clay rubbed on the edge of books lifts dirt away. At spas, people pay to have clay smeared on their faces to remove oils. Environmental cleanup crews use clay to absorb oil from spills.
Given the economic and environmental costs of other potential remedies for toxic algae, we have to continue to ask: Why not clay?
WHOI science writer Amy E. Nevala contributed to this article.
Slideshow

Slideshow
 CLEANING UP WITH CLAY—Scientist Mike Henry of the Mote Marine Laboratory sprays clay slurries into Florida’s Sarasota Bay while WHOI Postdoctoral Investigator Mario Sengco and colleagues in the other boat track the dispersal of the plume with water sampling devices. The researchers are investigating the use of natural clays as a potential tool to mitigate harmful algal blooms, or “red tides.” (Jim Cutter, Mote Marine Laboratory.)
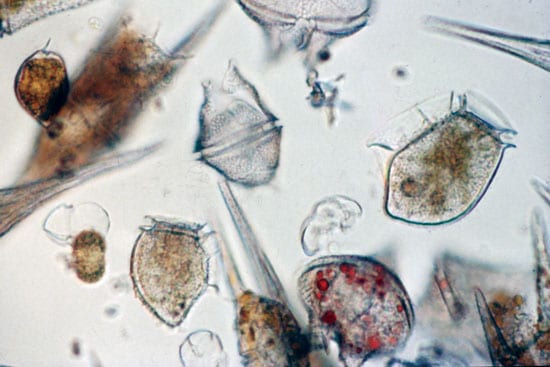
CLEANING UP WITH CLAY—Scientist Mike Henry of the Mote Marine Laboratory sprays clay slurries into Florida’s Sarasota Bay while WHOI Postdoctoral Investigator Mario Sengco and colleagues in the other boat track the dispersal of the plume with water sampling devices. The researchers are investigating the use of natural clays as a potential tool to mitigate harmful algal blooms, or “red tides.” (Jim Cutter, Mote Marine Laboratory.)- LITTLE PLANT, BIG PROBLEM—Harmful algal blooms are caused by species of tiny plants—phytoplankton—that produce potent chemical toxins. Fueled by periodic abundances of nutrients in the ocean, these algae multiply and proliferate until they can cover tens to hundreds of miles of coastal ocean. (Don Anderson, WHOI.)
- ON GUARD AGAINST ALGAE—In South Korea, fish farmers spread clay to ward off harmful algae that could devastate the crop in their pens. The results can be striking. After South Koreans began clay treatments, fishery losses fell from $100 million to $1 million per year. (National Fisheries Research and Development Institute, South Korea.)
- Removing harmful algal blooms with clay. (Illustration by Jack Cook, WHOI Graphic Services)
- TEXAS TWO-STEP—In an experiment in Corpus Christi, Texas, WHOI scientist Mario Sengco pumped algae-rich water into tanks and treated them with clay. Within hours, the sinking clay removed more than 70 percent of the algae. (Aishao Li.)
Related Articles
- Busting myths about HABs
- Are warming Alaskan Arctic waters a new toxic algal hotspot?
- Sargassum serendipity
- A dragnet for toxic algae?
- The Recipe for a Harmful Algal Bloom
- As Bay Warms, Harmful Algae Bloom
- Not Just Another Lovely Summer Day on the Water
- Setting a Watchman for Harmful Algal Blooms
- Dropping a Laboratory into the Sea