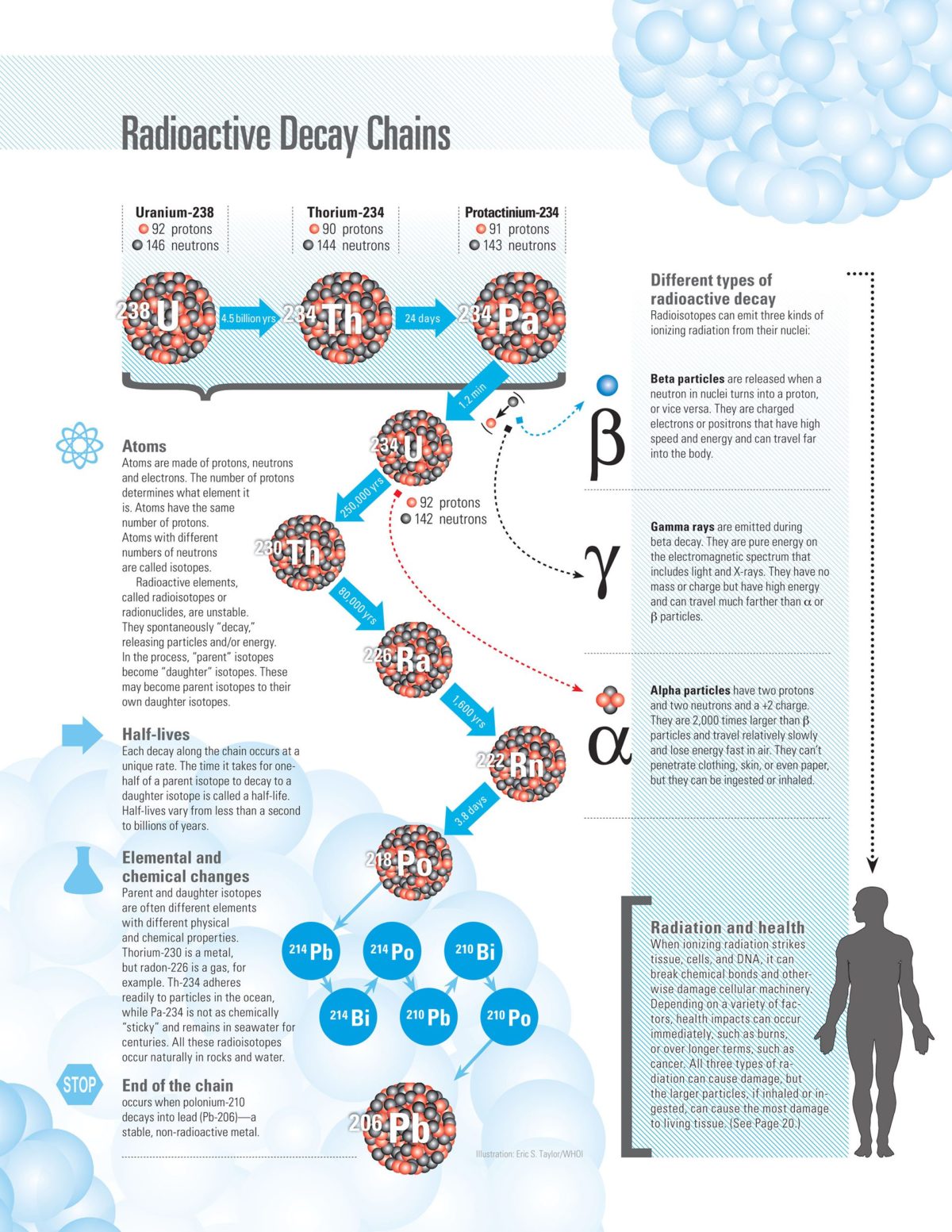
All radioisotopes—also called radionuclides—lose excess energy by emitting ionizing particles such as neutrons, protons, electrons, or photons. In the process, these so-called parent radioisotopes transform, or decay, into daughter isotopes containing different numbers of protons and neutrons. Daughters with the same number of protons are isotopes of the parent element; daughters with a different number of protons are actually different elements, with different chemical properties.
Each change along the way, follows a unique timetable, or half-life. The half-life of an isotope is the time it takes for one-half of the atoms in a given sample to decay. This daughter isotope can decay into another radioisotope, or the daughter isotope, that will continue the radioactive decay chain or into a stable element that ends the chain. (Illustration by Eric S. Taylor, © Woods Hole Oceanographic Institution)
Image and Visual Licensing
WHOI copyright digital assets (stills and video) contained on this website can be licensed for non-commercial use upon request and approval. Please contact WHOI Digital Assets at images@whoi.edu or (508) 289-2647.