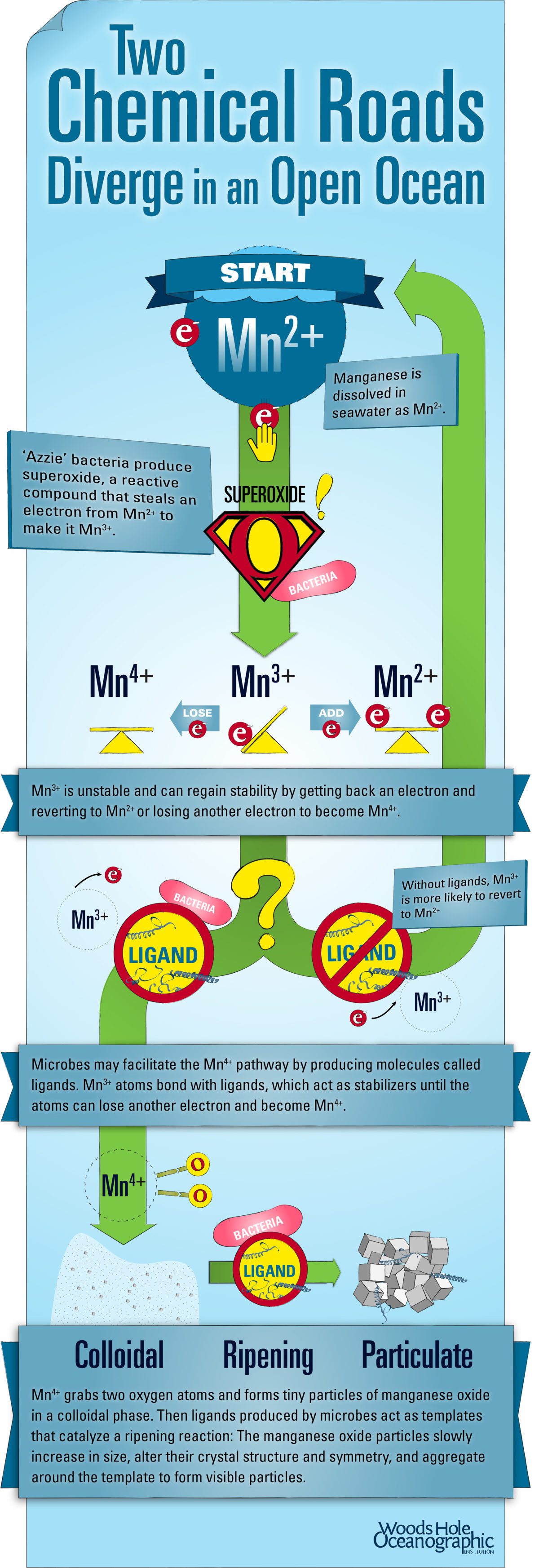
In the ocean and most other environments, manganese is present in seawater as a dissolved phase, Mn2+. To form manganese oxide minerals, manganese atoms need to give away two more electrons, oxidizing to Mn4+. In the process of oxidation, the manganese atoms grab a few oxygens, either from seawater molecules (H2O) or oxygen gas (O2) from air dissolved in seawater, and transform into a solid mineral. Here is the first place microbes play a role.
Previous research conducted by WHOI scientists found that many microbes produce and exude the compound superoxide—a highly reactive form of oxygen. Superoxide forms when your standard O2 molecule receives an additional electron to become O2. Superoxide is unstable and will react quickly with most molecules or atoms it encounters. In some cases, it will give back the electron it initially received, and in other cases, as with manganese, it will steal a second electron and bond to hydrogen atoms to become hydrogen peroxide (H2O2). This process oxidizes Mn2+ to Mn3+.
Like superoxide, Mn3+ is unstable and either wants to revert to Mn2+ or donate a second electron to become Mn4+. In most circumstances, Mn3+ reverts to Mn2+ because the reaction to form Mn4+ occurs more slowly. In natural systems, however, we hypothesize that manganese-oxidizing microbes help Mn3+ take the other route.
The microbes not only produce superoxide, which helps form Mn3+, they also produce and exude facilitating organic molecules, called ligands. Mn3+ atoms can bond with ligands until they can donate another electron and become fully oxidized to Mn4+.
The mysterious ripening’ template
WHOI scientists hypothesize that there’s a third piece to this puzzle: Biologically induced manganese oxides require an organic carbon template to grow around. Unlike nacre in mollusks, which forms around a specific polypeptide or protein, manganese oxides form around a range of proteins or sugars that may have similar properties. Beyond their role in facilitating electron transfer, the ligands or other organic molecules produced by Azzie may also serve in the crucial role of templates.
(Illustration by Eric S. Taylor, © Woods Hole Oceanographic Institution)
Image and Visual Licensing
WHOI copyright digital assets (stills and video) on this website can be licensed for non-commercial use upon request and approval. Please submit your request via our Media Request Form.
For assistance or accessibility accommodations, call (508) 289-2647.