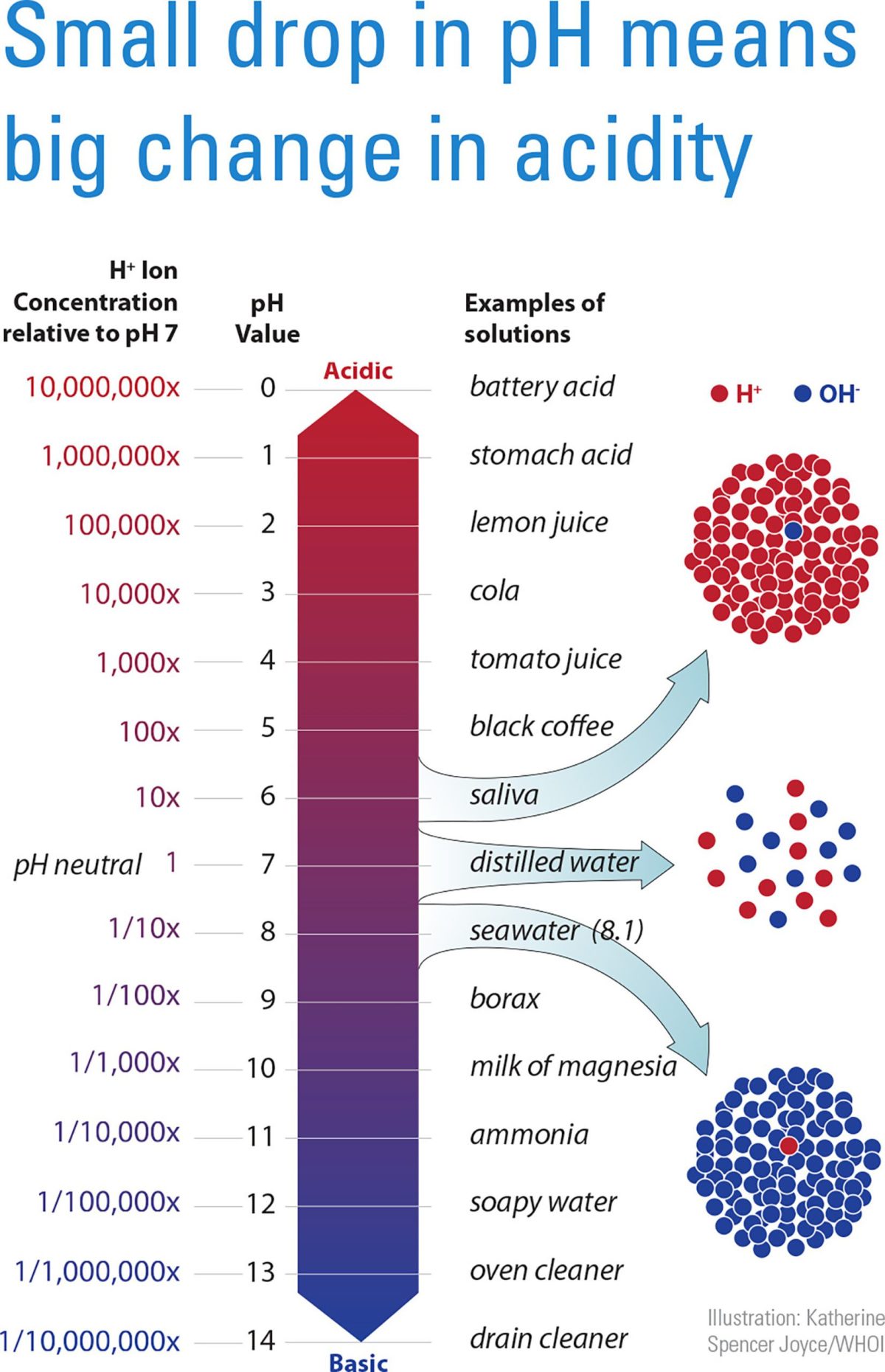
The pH scale, shown here, indicates the concentration of hydrogen ions (H+) in a liquid. Above pH7, a fluid is alkaline; below 7, it is acidic. Seawater is slightly alkaline, but has become more acidic in recent years due to increasing carbon dioxide in the atmosphere. Because the pH scale is logarithmic, a small change in pH represents a big change in relative acidity, which can have devastating effects on organisms that use calcium carbonate to build shells, spines, or other hard structures. Many scientists at WHOI are working to understand how ocean acidification affects marine organisms and, ultimately, human communities. (Illustration by Katherine Spencer Joyce, © Woods Hole Oceanographic Institution)
Image and Visual Licensing
WHOI copyright digital assets (stills and video) on this website can be licensed for non-commercial use upon request and approval. Please submit your request via our Media Request Form.
For assistance or accessibility accommodations, call (508) 289-2647.