 In situ flow cytometer being deployed off the WHOI dock
In situ flow cytometer being deployed off the WHOI dock
Robert J. Olson
Heidi M. Sosik
Biology Department
Woods Hole Oceanographic Institution
508 457-2000 x2565
x2311
and
Albert J. Williams 3rd
Applied Ocean Physics and Engineering
Woods Hole Oceanographic Institution
508 457-2000 x2725
One key to a better understanding of the biology, optics and geochemistry of the oceans is more detailed knowledge of the composition and characteristics of the suspended particles. At present, analysis of microscopic particles requires bottle sampling from a ship, which limits the resolution and duration of many studies. We have developed an instrument to analyze the optical properties (light scattering and fluorescence) of individual microscopic particles in situ and unattended, using established techniques of flow cytometry; the instrument is designed for moored operation. The flow cytometer utilizes a solid state diode-pumped laser which provides enough power to allow fluorescence measurements of single phytoplankton cells, but which has relatively low power-consumption.
We have taken two approaches in the design. In an attempt to increase reliability, we explored the use of a simple ducted flow to replace the mechanically complex hydrodynamic sample stream focussing of conventional flow cytometers. To use this simple flow system requires that we be able to recognize and reject signals from particles passing through the edges of the excitation spot; this was achieved by measuring light scattering from two orthogonal beams (from IR diode lasers) which define the sensing region. Figures from the poster presentation at the 1999 ASLO Meeting in Santa Fe, NM.
We found that the simple ducted flow approach worked well in laboratory tests with pure cultures. In natural samples of coastal seawater, however, the light scattering signals of the smallest phytoplankton cells (picophytoplankton, <2mm) were often significantly higher than expected due to additional scattering from other particles present in the measuring beam (but outside the intersection of the IR beams). Since we wished to measure the picoplankton as well as larger cells, we returned to a conventional flow cytometer design with injection of sample seawater into a filtered sheath stream, and a single focussed laser beam to measure light scattering and fluorescence. With this approach the performance of the instrument is similar to that of laboratory-based flow cytometry (e.g., Olson et al. 1993). Illustrations of the instrument and examples of in situ operation (at the LEO-15 mooring site off New Jersey) are shown below; additional results will be posted here after presentation at the ASLO meeting in February 2001 (Olson et al. 2001).
References
Olson, R.J., E.R. Zettler and M.D. DuRand. 1993. Phytoplankton
analysis using flow cytometry. In: Kemp, P.F., B.F. Sherr,
E.B. Sherr and J.J. Cole {Eds.}, Handbook
of Methods in Aquatic Microbial Ecology, Lewis Publ.,
777 pp.
Olson, R.J. and H.M. Sosik. 2000. An in situ flow cytometer for
the optical analysis of individual particles in coastal waters. (Abstract)
ASLO, Albuquerque, NM,
February 12-16, 2001.
Funds provided by NSF grants OCE-9416551 and 9907002, DOE grant DE-FG02-95-ER61982, and ONR grant N00014-93-1-1171.
 In situ flow cytometer being deployed off the WHOI dock
In situ flow cytometer being deployed off the WHOI dock
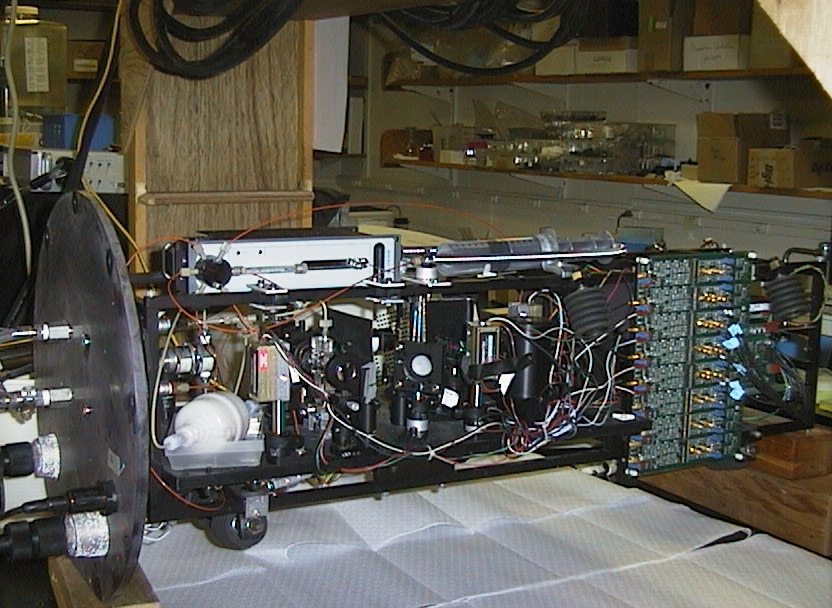 Flow cytometer with pressure housing removed
Flow cytometer with pressure housing removed
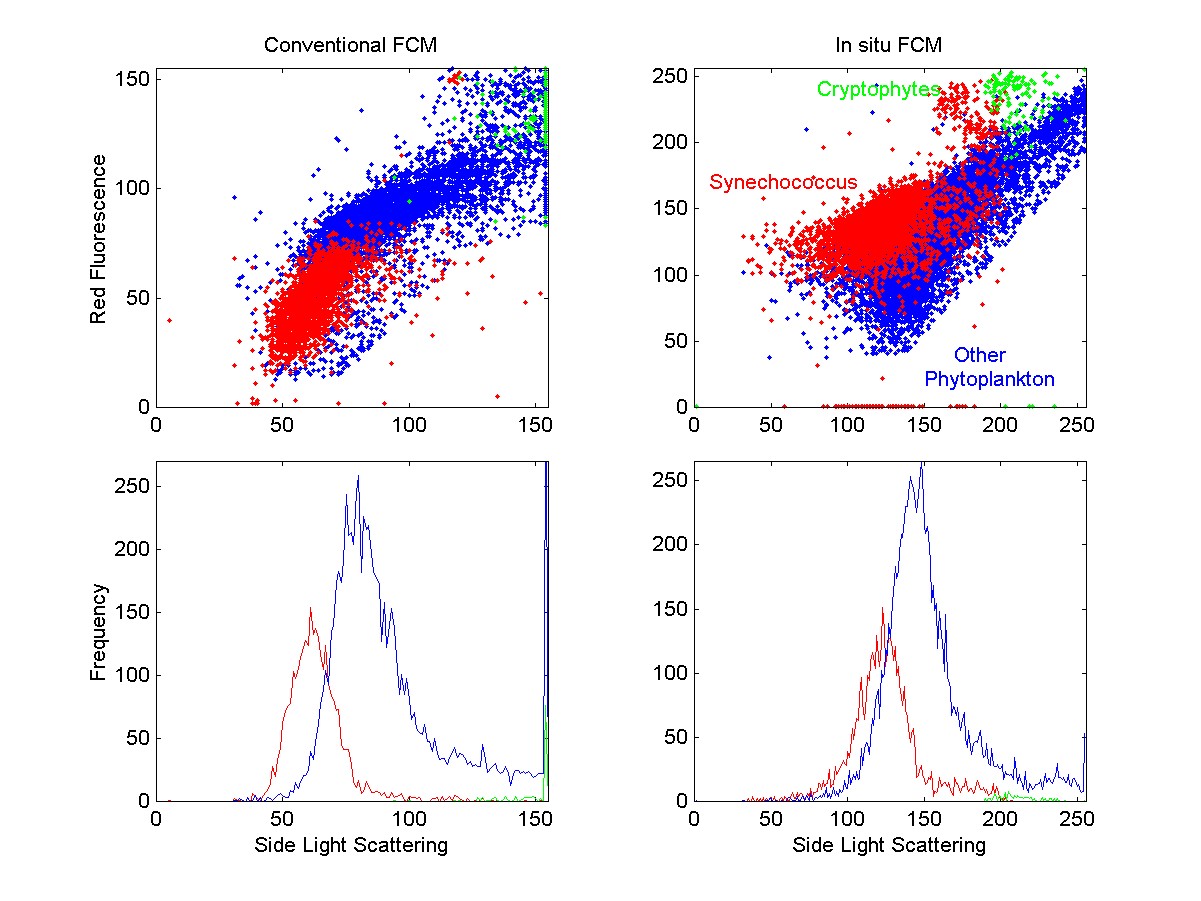
Flow cytometric signatures of phytoplankton in a
seawater sample from the WHOI dock (17 July 1999), analyzed in the lab
in parallel using the conventional and the in situ flow cytometers.
The raw data is plotted in log units to show cells ranging in size from
1 mm (Synechococcus) to >10 mm
(cryptophytes and other eukaryotes). Relative fluorescence of Synechococcus
and eukaryotic phytoplankton differ between the two instruments because
the excitation wavelength in the in situ flow cytometer (532 nm) excites
the major light-harvesting pigment of Synechococcus, phycoerythrin, more
efficiently than the 488 nm light used in the conventional instrument.
The concentration of cells in the three groups as determined by the two
instruments were similar (both total concentrations and proportions of
cells in the three groups agreed to within a few percent).
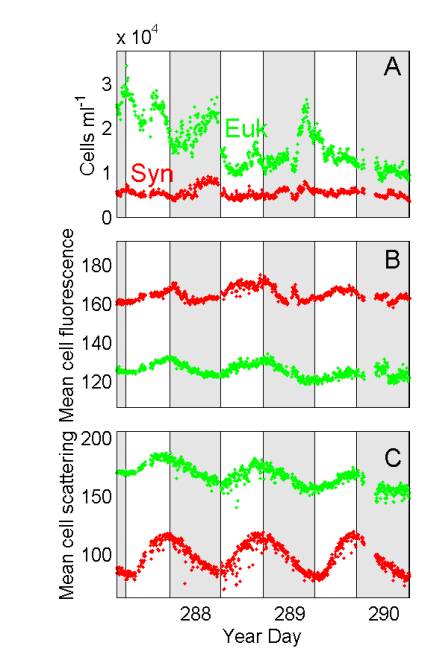 Phytoplankton characteristics measured by in situ flow cytometry at LEO-15,
as a function of time during a 3-day period in late October 2000.
Samples from 5-m depth were analyzed at 5-min intervals. Shaded bars
indicate night. Synechococcus cells were discriminated from eukaryotic
phytoplankton on the basis of phycoerythrin fluorescence.
Phytoplankton characteristics measured by in situ flow cytometry at LEO-15,
as a function of time during a 3-day period in late October 2000.
Samples from 5-m depth were analyzed at 5-min intervals. Shaded bars
indicate night. Synechococcus cells were discriminated from eukaryotic
phytoplankton on the basis of phycoerythrin fluorescence.
We expect changes in phytoplankton concentration
(A) at the mooring site to be influenced by a variety of processes, including
growth, grazing and sampling of different water masses due to tides and
currents. The observed patterns on the first two days of this series
(increases in cell numbers during the night) could reflect phased cell
division. This conclusion is supported by the patterns in mean optical
properties of the phytoplankton populations, which are expected to be much
less sensitive than cell numbers to grazing and advection. Mean cell
fluorescence (B) of Synechococcus (phycoerythrin) and of eukaryotic phytoplankton
(chlorophyll), varies systematically, increasing during the day and decreasing
during the night. The patterns in light scattering (C), which is
related to cell size, are even stronger evidence of cell growth during
the day and division during the night.