

Trichodesmium
Photosynthetic fixation of CO2 in the oceans accounts for approximately half of total global primary production. Cyanobacteria, including species of Synechococcus, Prochlorococcus, Trichodesmium, and Crocosphaera, are prominent constituents of the marine biosphere that contribute significantly to the “biological carbon pump”. The factors that control the growth of these cyanobacteria directly impact not only the carbon pump, but also the global nitrogen cycles through the activity of diazotrophs (i.e., Trichodesmium and Crocosphaera). The introduction of this new nitrogen to the euphotic zone is significant since it allows further CO2 fixation by the remaining non-diazotrophic phytoplankton community. Observations from the tropical and subtropical North Atlantic indicate that species of the macroscopic (0.5-4mm) marine cyanobacterium, Trichodesmium are the most significant cyanobacterial primary producers (fixing ~165mg CO2/m2/day). Furthermore, in this region Trichodesmium introduces the largest fraction of new nitrogen to the euphotic zone (~30mg N/m2/day), a value that exceeds the estimated flux of nitrate across the thermocline. In addition to the Atlantic, studies indicate that Trichodesmium is also a significant source of new nitrogen in the tropical Pacific Ocean and likely the Indian Ocean as well. In short, cyanobacteria are significant primary producers at the base of the marine food chain, with the genus Trichodesmium being particularly important in the tropical and subtropical oceans due to both its high abundances and N2-fixation rates.
Five morphologically distinct species of Trichodesmium have been described in the literature. They are, Trichodesmium tenue, T. erythraeum, T. thiebautii, T. contortum, and T. hildebrandtii. T. erythraeumis the best-studied species in both cultures and field populations. Little is known about how these species differ physiologically, and what geochemical and physical factors drive Trichodesmium species diversity in situ. The dearth of physiological data is due in part to the difficulties associated with culturing Trichodesmium. Although there are several strains of T. erythraeum maintained in other laboratories, we have developed techniques to maintain the five Trichodesmium spp. in culture at the Woods Hole Oceanographic Institution. These techniques have been extended further to render several cultures axenic. This culture collection will provide material for our molecular and physiological analyses.
Recently the Department of Energy/Joint Genome Institute (DOE/JGI) has completed the first draft of the genome of T. erythraeum. We are currently in the process of initiating the annotation efforts and developing molecular projects that utilize this genomic information.
Projects
Photosynthetic fixation of CO2 in the oceans accounts for approximately half of total primary production. A significant component of this primary production is due to the growth of oceanic cyanobacteria, such as the diazotroph Trichodesmium. For example, in the tropical and subtropical North Atlantic, Trichodesmiumspp. are thought to be the most significant primary producers. Additionally, in this region Trichodesmium introduces the largest fraction of new nitrogen to the euphotic zone (~30mg N/m2/day), a value that exceeds the estimated flux of nitrate across the thermocline. Although there are many biogeochemical factors that may impact Trichodesmium productivity, and N2fixation (e.g. nutrients, light, temperature), recent studies have emphasized the critical importance of P and Fe bioavailability. Definitively demonstrating P or Fe limitation of Trichodesmium in situ is not trivial and research often relies on either extrapolation of culture data from one species to field populations, or the inference of physiological condition from geochemical data. To increase our understanding of how the bioavailability of these elements may constrain primary production and N2 fixation we are developing specific molecular diagnostics for Fe and P limitation in Trichodesmium.
In axenic cultures we have determined that P-stressed Trichodesmium colonies induce the enzyme PhoA and Fe-stressed colonies express the protein IdiA. We have also demonstrated that we can detect PhoA activity and IdiA expression in field populations. We are initiating laboratory studies to determine how these diagnostics correlate with decreases in both Trichodesmium productivity and N2 fixation. After our culture work we further propose to apply these diagnostic tools to field populations. This project is a collaborative effort between Eric Webb, Sonya Dyhrman, John Waterbury, and Jim Moffett.
Fe stress response of axenic Trichodesmium IMS101 (taken from Webb, E.A et al AEM 2001). (A) Coomassie stained SDS-PAGE gel from cultures grown under Fe replete (+Fe) or omitted (-Fe) conditions. Arrows denote differentially expressed proteins and their approximate molecular weight. (B) Western blots detecting IdiA expression in Fe stressed axenic Trichodesmium IMS101 with polyclonal Synechococcus PCC6301 anti-IdiA antiserum and pre-immune serum. An arrow identifies the IdiA protein. Open ocean Synechococcus WH7803 is presented as a positive control for the blot.
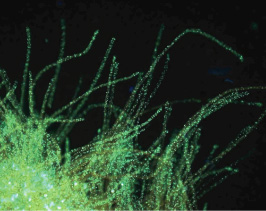
Epifluorescent micrograph of aTrichodesmium thiebautii sample from the Western North Atlantic assayed for phosphate stress, using our alkaline phosphatase (PhoA) detection technique (outlined in detail in Dyhrman et al Limnol. Oceanogr. 2002). Red/orange phycoerythrin autofluorescence is visible in the filamnets, while the green fluorescent product is due to PhoA activity.
![]()
Back
to top
![]()
Comments
and questions please mail to ewebb@whoi.edu
Last
updated 09/02