

Tests of a new sampler for collecting aerosols from ocean buoys are showing promising results. The idea of using buoys as platforms for more precise sampling of continental dust falling on the oceans sprang from seeing large quantities of reddish brown Saharan dust adhering to buoy-mounted meteorological sensors in the ocean off northwest Africa. The deployment of meteorological and physical oceanographic instruments from moored buoys is a well established and an important tool in studies of marine meteorology, atmosphere-ocean interactions and upper ocean physics (Rudnick et al., 1997; Weller et al., 1990). An emerging field of research in oceanography is centered on developing analytical instruments for the chemical, biological and optical measurements of seawater under moored buoys (Dickey et al., 1997). Research on aerosols, in particular, and atmospheric chemistry, in general, has not been previously attempted from buoys.
Aerosols, an assembly of solid or liquid particles suspended in air, range in size from 0.001 to over 100 µm. The most common types of aerosols are mineral dust, seasalt particles, organic particles, soot, fine cloud condensation nuclei particles, and clouds. Natural aerosols are generated by oceans, desert, soil, vegetation, forest fires, and volcanoes. Aerosols also come from anthropogenic activities, such as gasoline, oil, coal and charcoal combustion, biomass burning, cement production, smelting and agriculture. Aerosols also form when gases are converted to fine particles in the troposphere. The formation of non- seasalt sulfate (NSS) aerosols from the oxidation of gaseous sulfur dioxide (SO2) is the most prominent example.
Certain marine phytoplankton release gaseous dimethylsulfide (DMS) which is transported across the ocean/atmosphere boundary to the marine boundary layer where atmospheric chemical reactions convert DMS to NSS particles. This combination of biology, biogeochemistry, physical transport and atmospheric chemistry leads to the oceans being the major source of NSS aerosols which affect climate as they are important in the formation of clouds. The quantitative relationship between phytoplankton productivity and DMS emissions to the marine boundary layer remains elusive (Andreae and Crutzen, 1997). Seasalt aerosols are involved in complex heterogeneous reactions which are thought to deplete the ozone concentrations in the marine boundary layer of the polar regions. Dust and combustion products can be important sources of trace metals and anthropogenic organic compounds to lakes, estuaries and oceans. The Iron Hypothesis is built around the argument that phytoplankton production in large regions of world’s oceans is limited by iron which is supplied, in the main, by continental dust (Duce and Tindale, 1991; Martin et. al., 1991) Dust is transported long distances from Asia and Africa across large expanses of the North Pacific and Atlantic Ocean (Arimoto et. al., 1992; Prospero et al., 1989) and deposited on the oceans in short episodic events and in strong seasonal pulses. The episodic nature of aerosol transport to and from the oceans is a real challenge to sample in a comprehensive manner from ships or aircraft. Shipboard studies of upper ocean biology and chemistry have rarely coincided with a dust deposition event. Hence, the enhancement of biological production by dissolved iron released from a dust event remains a hypothesis. Confirmation of the Iron Hypothesis requires long-term measurements of dust input along with simultaneous measurements of the physical, chemical and biological variability of the upper oceans. Sampling systems 3-10 m above the sea surface on buoys are well positioned to study processes associated with aerosol deposition and production near the ocean/atmosphere boundary. In contrast to ships, buoys can stay on station for 3-12 months, thus providing a less expensive and alternative platform for long-term time-series research and monitoring programs and for short-term field experiments. Buoys can be moved to strategic research regions. Aerosol data from islands cannot be accurately related to the deposition of dust and the production of seasalt, DMS and NSS aerosols taking place hundreds of miles away on the oceans. Having buoy-mounted aerosol samplers remotely controlled from shore would open up new research possibilities. Sampling could be based on satellite pictures of dust clouds, volcanic eruptions, biomass burning or increased biological productivity.
We tested our sampler for seven months in the field: four months on the AEROCE (Atmosphere/Ocean Chemistry Experiment) tower in Bermuda and three months on a buoy moored off Woods Hole (Figure 1). The tower and buoy were instrumented for wind speed and direction, rate of precipitation and rain detection. A wireless two-way communications modem was added to the buoy system, thus enabling us to monitor the sampling progress, to remotely change the sampling parameters, and to download the meteorological data.

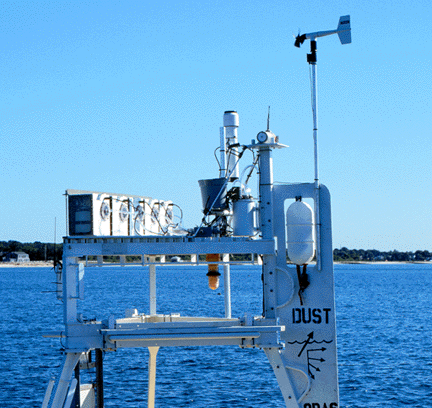
| Photographs of WHOI's Autonomous Aerosol Sampler in Vineyard Sound off Woods Hole MA in August 1997. The top figure shows the five white filter modules mounted across the front of a 3 meter discus buoy. The vane keeps the air inlets ( dark circular area on each filter module) into the wind. The bottom figure is a side view showing the control module ( white cylinder behind and below the gray bucket), the electrical and vacuum lines leading from the control module to the back of the five filter modules, the rain gauge ( tall white cylinder), the wind speed/direction sensor mounted on top of the vane and the RF wireless modem with its antenna. The rain detector is mounted in the gray bucket. On the deck of buoy in the top figure is a fiber optics pCO2 sensor system developed in the laboratory of David R. Walt at Tufts University; the sensor is submerged about 1 m below the ocean surface. |
Each filter module consists of an inlet, a motorized ball valve, an aerosol filter holder, a vacuum port and a local microcontroller. A polypropylene ball valve protects the filter from contamination before and after the air filtering step and seals the inside of the filter module from inclement weather. The polypropylene aerosol holder screws into the back of the ball valve, allowing the filters to be loaded into the filter modules without being touched. The filter holder will accept any type of 47 mm diameter filter. The choice of filter material and pore size retention is determined by the demands of specific projects. Filter packs with different types of filters in series or with gas scrubbers could be added to the filter modules. The airflow path is all plastic to limit contamination with respect to the measurement of trace metals. We opted for a simple funnel/tube design for the inlet which keeps the airflow path to the filter short (10 cm) and of uniform diameter (38 mm). The vane on the buoy keeps the inlets of the filter modules into the wind except under low (< 2 m/s) speeds.
Software controls the duration and sequence of filtering through the five modules and monitors the flow rate and the meteorological conditions. Moreover, the software can respond intelligently to varying conditions of the atmosphere and oceans. For example, filtering can be halted during periods of rain and high winds, which cause breaking waves that could lead to contamination. Sectoring capability is a powerful feature of our system. That is, aerosol sampling can be carried out over pre-set ranges of wind speed and direction. These ranges can be modified on the fly using a wireless or satellite modem. A sampling strategy based on different ranges of humidity, sunlight, or temperature could be arranged by adding the corresponding sensors. Studies involving photochemistry might benefit from setting up a day/night sampling cycle.
Our system can operate in a duty cycle mode for a minimum of 3-4 months with filtering rates of 10-15 lpm (14-22 m³/day). Many trace elements (e.g., iron, lead, zinc) can be measured with a 50 µg sample of dust. This translates into 2 -3 days of filtering air with a dust concentration equal to the annual mean Bermuda value of 1 µg/m³. With our next generation of smaller filter modules and/or filter carousels, we will be able to collect 20-50 aerosol samples per deployment, leaving considerable flexibility in the sampling strategy.
The three month time-series Fe concentrations of the ten WHOI samples range from 0.02 to 0.6 µg Fe/m³ (Figure 2) and agree well with published data from AEROCE Bermuda samples from previous years (Arimoto et al., 1992). Both the WHOI and AEROCE samplers picked up the large pulse of particulate Fe in early October; this aerosol's reddish brown color indicates an African origin. The direct comparison between three WHOI filters and the corresponding AEROCE samples at Bermuda are very encouraging as the Fe concentrations compare to within 10-15% over a wide range of values. This agreement is more remarkable when considering that the comparison involves different time and volume scales of sampling.
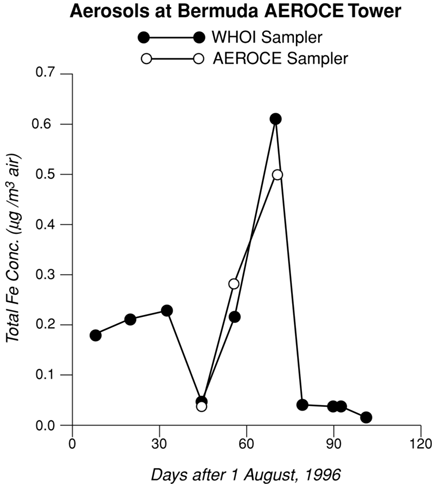
| Time-series data for total iron concentration of aerosols collected on the Bermuda tower: a comparison of WHOI's Autonomous Aerosol Sampler and AEROCE’s high volume sampler. |
Future improvements to our sampler will include a reduction in the size and weight of the filter modules and the development of a carousel system capable of generating a time- series set of fifty aerosol-embedded filters from a single compact module. Real-time data on the variability and composition of aerosols, selected trace gases and rainwater, all obtained from buoy-mounted sensors, would prove valuable for the studies of oceanography, atmospheric chemistry and climatology. For example, micro-gas chromatography is being developed for in-situ DMS analyses on buoys (John Dacey, per. comm.). Ozone, a gas of immense significance in atmospheric chemistry, has the potential to be measured in real-time from buoys. Systems adaptable to buoys are being developed scavenging for SO2 and DMS from air. It is technologically feasible to measure the rainwater concentrations of nutrients and Fe in real time from a buoy.
The authors have recently been funded to design, build and test sensors which can measure aerosol concentrations in real-time from ocean buoys. X-ray fluorescence spectroscopy, a technique employed on the Mars rover, offers great promise for the in situ elemental (Fe, K, Ca, Mg, Cl and S) analysis of aerosols from buoys. By using this non- destructive technique, applied to the analyses of aerosols embedded on filters, we can distinguish between mineral dust, seasalt and perhaps NSS.
One can envision a remotely controlled field laboratory in which buoy-mounted sensors monitor the response of upper ocean biology and chemistry to pulses of dust. Using real- time aerosol and rainwater Fe concentration data, researchers could trigger buoy-mounted water sensors, water samplers and sediment traps. These could measure light scattering, chlorophyll, nutrients, dissolved oxygen and collect water and ocean particles for the measurements of Fe, trace metals, thorium isotopes, organic carbon, phytoplankton species and dust. Similarly, time-series air data on ozone, DMS and NSS from buoys could be coupled with in-water measurements of phytoplankton production, chlorophyll, turbulence and nutrients to study the biogeochemical cycle of sulfur above and below the ocean/atmosphere boundary. Lastly, buoys provide a well positioned platform to gather better data on the production of seasalt aerosols as a function of wind stress, a parameter of importance to models of aerosol distribution and optical scattering over the oceans.
We encourage readers interested in exploring collaborative projects on analytical methods development and in innovative uses of surface moorings for studies of atmospheric chemistry to contact the first author (esholkovitz@whoi.edu).
Andreae M. O. and P. J. Crutzen. Atmospheric aerosols: biogeochemical sources and role in atmospheric chemistry, Science, 276, 1052, 1997.
Arimoto, R., R. A. Duce, D. L. Savoie, and J. M. Prospero. Trace elements in aerosol particles from Bermuda and Barbados: concentrations, sources and relationships to aerosol sulfate, J. Atmos. Chem., 14, 439, 1992.
Dickey T. D., D. Frye, H. W. Jannasch., E. Boyle. and A. H. Knap. Bermuda sensor system testbed, Sea Technology, April, 81, 1997
Duce, R. A. and N. W. Tindale. Atmospheric transport of iron and its deposition in the ocean. Limnol. Oceanogr., 36, 1715, 1991.
Duce, R. A., P. S. Liss, J. T. Merrill, E. L. Atlas, P. Buat-Menard, B. B. Hicks, J. M. Miller, J. M. Prospero, R. Arimoto, T. M. Church, W. Ellis, J. M. Galloway, L. Hansen, T. D. Jickells, A. H. Knap, K. H. Reinhardt, B. Schneider, A. Soudine, J. J. Toko, S. Tsunogai, R. Wollast, and M. Zhou. The atmospheric input of trace species to the world ocean. Global Biogeochem. Cycles, 5, 193, 1991
Martin, J. H., R. M. Gordon, and S. E. Fitzwater. The case for iron. Limnol. Oceanogr., 36, 1793, 1991
Prospero, J. M., M. Uematsu, and D. L. Savoie. Mineral aerosol transport in the Pacific Ocean. in Chemical Oceanography, (eds. J. P. Riley and R. Chester) Academic Press, 10, 187, 1989
Rudnick D. L., R. A. Weller, C. C. Eriksen, T. D. Dickey, J. Marra and C. Langdon. Moored instruments weather Arabian Sea Monsoons, yield data. Eos, Trans., AGU, 78, 117, 1997
Weller, R. A., D. L. Rudnick, R. E. Payne, J. P. Dean, N. J. Pennington, and R. P. Trask. Measuring near-surface meteorology over the ocean from an array of surface moorings in the subtropical convergence zone, J. Atmospheric and Oceanic Technology, 70, 85, 1990.
Wu J. and E. A. Boyle, Lead in the western North Atlantic Ocean: Completed response to leaded gasoline phaseout, Geochim. Cosmochim. Acta, 61, 3279, 1997.
Last Modified: 20 Apr 98
by Geoff Allsup