Syllabus
Mak Saito - Fall 2022 |
|
 |
 |
Enlarge Image
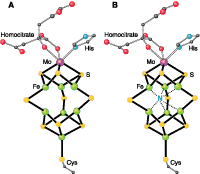 |
Nitrogen in the middle. Model of the FeMo cofactor of the nitrogen-fixing enzyme nitrogenase (A) before and (B) after the Einsle et al. report (1). This report presents a high-resolution structure of the MoFe protein of nitrogenase, which contains the FeMo cofactor. The new work reveals a previously unrecognized interstitial atom in the FeMo cofactor that may possibly be nitrogen (blue N). Carbon, gray; iron, green; sulfur, yellow; molybdenum, purple; oxygen, red; and nitrogen, blue. (From Smith et al., Science 2002)
|
 |
| Related Links |
|
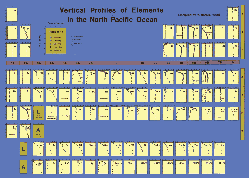
| OverviewMarine Bioinorganic Chemistry is an emerging field that examines the distribution and processes that affect inorganic elements throughout the natural environment, combining knowledge from the fields of trace metal biogeochemistry and bioinorganic chemistry. A broad scale range will be considered from subcellular biochemical levels involving metalloproteins and metal-binding molecules to global and geological scales to relevant to global biogeochemical cycles. This course aims to cover major research areas and key chemical and biological principles, quantitative chemical aspects, survey new research avenues, describe key methodologies, and to provide opportunities for class discussion on subjects that intersect with policy.
Course Outline
Lecture Topics:
- Introduction to trace metal biogeochemistry and bioinorganic chemistry
- Categories of trace elements
- Irving Williams series, concepts of hardness and softness
- Metal speciation and analytical methodologies
- Metalloenzymes and their role in marine biogeochemistry
- Specific elemental biogeochemistries (Mn, Al, Pb, Co, Zn, Cd, Cu)
- Free ion/metal prime models and algal uptake kinetics
- Phytoplankton limitation and colimitations
- Mercury and toxic metal biogeochemistry
- Iron biogeochemistry (limitation, light colimitation, redox, speciation, uptake mechanism, colloids, and policy)
- Hydrothermal vents biogeochemistry
- Trace elements and isotopes in studies of deep time and the ancient ocean
- Use of bioinformatic resources in marine biogeochemistry and biochemistry
- Element use in human society and industrial ecology
Discussion Topics:
- Iron fertilization, geoengineering, carbon sequestration
- Ideas and creativity in science
- Industrial ecology and human sustainability
Course Work:There will be four problem sets throughout the semester (10% of course grade each), a mid-term exam (10%), a semester classroom research educational project culminating in brief summary of a protein/enzyme using the Ocean Protein Portal that will be posted on a public wiki (20%), a research paper/proposal (>6 pages, 20%), and a 10 minute class presentation related to the paper to be presented at the end of the semester (10%). Paper topics are to be chosen by students, in communication with the instructor to point to and discuss availability of literature sources. There will also be three classroom discussions/debates on selected readings (labeled for discussion) and topics.
|
|
