|
 |
|
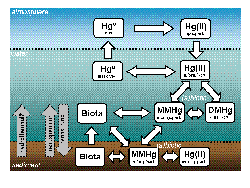
Enlarge Image The biogeochemistry of mercury in the atmosphere and ocean.
|
 |
| Related Links |
|
| Human Health and Mercury Biogeochemistry
Mercury (Hg) is a toxic trace metal with both natural and
anthropogenic sources to the environment (Fitzgerald and Clarkson, 1991). The primary exposure route for humans is the
consumption of fish and fish products, and the majority of the consumed fish
species are marine (NRC, 2000). Studies of the secular change of Hg in the
environment using a variety of natural archiving media have indicated that the amount
of Hg entering ecosystems has increased by 2-5x (avg. ca. 3x) since the
Industrial Revolution (e.g., Jensen and Jensen, 1990; Swain et al., 1992; Monteiro and Furness,
1997; Lamborg et al., 2002; Schuster et al., 2002). Subsequently, numerous
local, state, national and international authorities have recognized Hg as a
pollutant of special concern (e.g., JECFA, 2000; NAS, 2000). This is highlighted by the adoption of fish
consumption advisories for fresh and saltwater fisheries around the United States
and Europe (e.g., USFDA, 2001; Schober et
al., 2003).
Hg is found, under typical ambient conditions, in three
chemical forms: elemental Hg (Hgº), divalent ionic Hg in a variety of inorganic
and organic complexes (in total, Hg(II)) and methylated forms (mono- and
dimethylHg, MMHg and DMHg, respectively). As illustrated in the figure below, these
species groups are intricately linked together. Formation of the methylated
forms is of particular interest, as it is these more lipophilic forms that are
especially toxic and bioaccumulative.
In our lab, we are investigating the biological, physical and chemical factors that affect the movement of Hg between these forms...not only in the ocean, but in lakes and the atmosphere as well.
- Fitzgerald, W.F., Clarkson, T.W., 1991. Mercury and
monomethylmercury: present and future concerns. Environ Health Persp 96,
159-166.
- JECFA (2000).
Safety Evaluation of Certain Food Additives and Contaminants. WHO Food
Additives Series: 44; Methylmercury. Geneva,
Switzerland, 53rd Joint FAO/WHO Expert Committee on Food Additives
(JECFA), World Health Organization: http://www.inchem.org/documents/jecfa/jecmono/v44jec13.htm.
- Jensen, A.,
Jensen, A., 1990. Historical deposition rates of mercury in Scandinavia estimated by dating and measurement of mercury in cores of peat bogs.
Water Air and Soil Pollution 56, 769-777.
- Lamborg,
C.H., Fitzgerald, W.F., Damman, A.W.H., Benoit, J.M., Balcom, P.H., Engstrom,
D.R., 2002. Modern and historic atmospheric mercury fluxes in both hemispheres:
Global and regional mercury cycling implications. Gl Biogeochem Cycles 16,
1104, doi: 10.1029/2001GB001847.
- Monteiro,
L.R., Furness, R.W., 1997. Accelerated increase in mercury contamination in
north Atlantic mesopelagic food chains as indicated by time series of seabird
feathers. Environ Toxicol Chem 16, 2489-2493.
- NAS (2000). NationalAcademy of Sciences Report: Toxicological Effects of
Methylmercury. C. R.A. Goyer, NationalAcademy Press: pp.
344.
- NRC (2000). National
Research Council Report: Toxicological effects of methylmercury. Washington, D.C., National Academic Press.
- Schober,
S.E., Sinks, T.H., Jones, R.L., Bolger, P.M., McDowell, M., Osterloh, J.,
Garrett, E.S., Canady, R.A., Dillon, C.F., Sun, Y., Joseph, C.B., Mahaffey,
K.R., 2003. Blood mercury levels in U.S. children and women of childbearing age, 1999-2000.
Journal of the American Medical Association 289, 1667-1674.
- Schuster,
P.F., Krabbenhoft, D.P., Naftz, D.L., Cecil, L.D., Olson, M.L., Dewild, J.F.,
Susong, D.D., Green, J.R., Abbott, M.L., 2002. Atmospheric mercury deposition
during the last 270 years: A glacial ice core record of natural and
anthropogenic sources. Environ Sci Technol 36, 2303-2310.
- Swain, E.B.,
Engstrom, D.R., Brigham, M.E., Henning, T.A., Brezonik, P.L., 1992. Increasing rates
of atmospheric mercury deposition in midcontinental North America. Science 257, 784-787.
- USFDA (2001).
Mercury levels in seafood species. Washington, D.C., U.S. Food and Drug Administration, Center for Food Safety and Applied
Nutrition, Office of Seafood.
Last updated: March 9, 2009
|

